Article contents
Redox-responsive supramolecular polymer based on β-cyclodextrin and ferrocene-decorated main chain of PAA
Published online by Cambridge University Press: 06 October 2015
Abstract
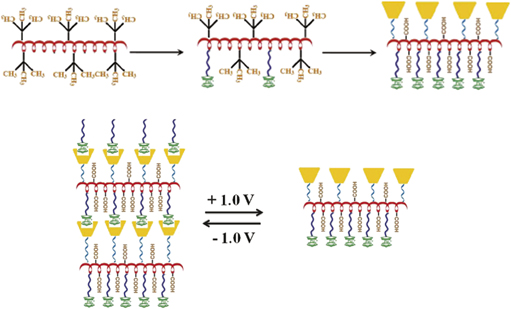
Herein, we present a method for introducing β-cyclodextrin (β-CD) and Ferrocene (Fc) into the main chain of poly(acrylic acid) (PAA) to fabricate a novel supramolecular polymer, which was investigated by Fourier transform infrared spectroscopy and 1H Nuclear Magnetic Resonance (1H NMR). This polymer can self-assemble into interesting nanoparticles in aqueous solution. The redox-responsive Fc–CD host–guest interactions endow these nanoparticles with unique self-degradable and self-healable features under redox potential control. This redox behavior of the supramolecular polymer would open up an approach for redox-controlled biological materials with great application potential.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
Footnotes
Contributing Editor: Tao Xie
References
REFERENCES
- 7
- Cited by


