Article contents
Plasmon-band subpeak and oxidation of solar-control LaB6 nanoparticles
Published online by Cambridge University Press: 13 July 2016
Abstract
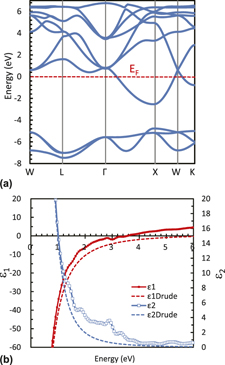
Near-infrared (NIR) absorption in solar-control LaB6 nanoparticles (NPs) is derived from the localized surface plasmon resonance (LSPR) at 1.3 eV, and accompanies an unclarified subpeak at 1.8 eV. As an origin of this subpeak, a disk-like particle shape of LaB6 NP has recently been proposed, besides the previously-proposed, milling-derived LaO phase. A series of heating experiments at 200–850 °C in air for LaB6 NPs pulverized with different media beads have been made, followed by x-ray diffraction and transmission electron microscopy observations, to clarify that LaB6 NPs oxidizes to amorphous phases B2O3 and La–B–O at 450–600 °C, and crystallize to LaB3O6 at 650–750 °C, without forming LaO or La2O3. Dielectric functions of LaO have been derived by first-principles calculations using sX-LDA, and Mie scattering calculations have been made for various sizes, shapes, and the ensembles, showing that LaO NPs, if existed, should exhibit an excessively-broadened absorption band with a blunt LSPR peak at 2.1 eV buried in several interband-transition absorptions at 1.2–4.0 eV. These analyses confirm that the observed 1.8 eV subpeak could not originate from LaO and support the nonspherical shape of NPs as the origin of the subpeak.
- Type
- Focus Section: Reinventing Boron Chemistry and Materials for the 21st Century
- Information
- Journal of Materials Research , Volume 31 , Issue 18: Focus Section: Reinventing Boron Chemistry for the 21st Century , 28 September 2016 , pp. 2780 - 2788
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 8
- Cited by



