Published online by Cambridge University Press: 22 December 2014
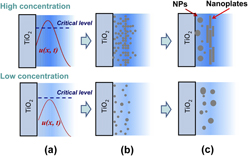
Using nanoparticulate TiO2 films, the photocatalytic growth of Ag nanoparticles (NPs) in the AgNO3 aqueous solution has been studied in terms of reduction, nucleation, and coalescence. It was proved that Ag primary particles were formed in a growth time of <1 s after the photocatalysis started. The growth dynamics was found to be critical for isotropic and anisotropic growth of Ag NPs, depending on the AgNO3 concentration and surface properties of TiO2 films. In the AgNO3 solutions of ≤300 mg/L, the isotropic growth dominates the growth dynamic behavior, producing irregularly spherical Ag NPs. In the AgNO3 solutions of ≥400 mg/L, the increased reduction rate promotes the formation of Ag nanoplates in the product. Ostwald ripening and oriented attachment were suggested to be the mechanisms dominating the isotropic and anisotropic growth, respectively. A photocatalytic growth model of Ag NPs was proposed by taking Ag atom and Ag+ ion diffusion into consideration. The plasmonic properties of the Ag–TiO2 films were studied in terms of extinction, surface enhanced Raman scattering, and fluorescence enhancement.
To send this article to your Kindle, first ensure no-reply@cambridge.org is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about sending to your Kindle. Find out more about saving to your Kindle.
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox.
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive.