Article contents
Facile room temperature synthesis of Ag@AgBr core–shell microspheres with high visible-light-driven photocatalytic performance
Published online by Cambridge University Press: 18 February 2015
Abstract
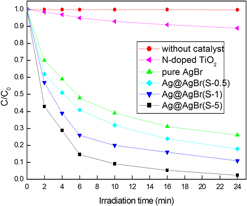
The uniform Ag@AgBr core–shell microspheres were synthesized by a very facile wet-chemical route in aqueous solution, including a reduction process to prepare sphere-like Ag core and a deposition process to synthesize AgBr shell. X-ray diffraction, x-ray photoelectron spectroscopy, field emission scanning electron microscopy, and high-resolution transmission electron microscopy results confirmed the formation of Ag@AgBr core–shell heterostructures which had been achieved by this simple method. Field emission scanning and high-resolution transmission electron microscopy results of the as-synthesized Ag@AgBr composite revealed that AgBr particles were deposited on the surface of sphere-like Ag core. Under visible-light (λ > 420 nm) and real sunlight irradiation, the as-synthesized Ag@AgBr samples exhibit high activity and good stability for the photodegradation of Rhodamine 6G (R6G) in water. The present work suggests that the as-synthesized Ag@AgBr core–shell microsphere can be applied as a visible light-activated photocatalyst in efficient utilization of solar energy for treating water polluted by some chemically stable azo dyes in environment. The enhanced photocatalytic performance of the as-synthesized Ag@AgBr composite might be attributed to accelerated separation efficiency of electron–hole pairs on the interface of the Ag@AgBr hybrids and improved visible-light absorption abilities when AgBr is coupled with Ag.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 8
- Cited by


