Published online by Cambridge University Press: 29 May 2017
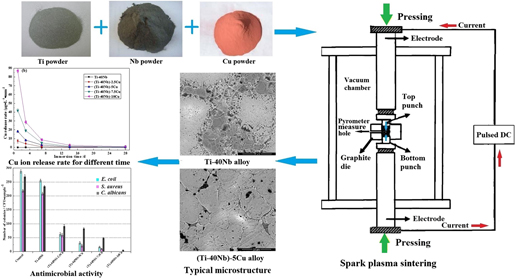
A superelastic Ti–40Nb alloy enhanced with Cu element (0, 2.5, 5, 7.5, and 10 wt%) was synthesized by a spark plasma sintering method to obtain biomaterials with an antimicrobial effect. The microstructure results showed that β phase was the main phase in (Ti–40Nb)–Cu alloys while Ti2Cu was synthesized with the Cu addition above 5 wt%. (Ti–40Nb)–Cu alloys exhibited high compressive strength over 1693.08 MPa, high yield strength of 1140.26–1619.14 MPa, low elastic modulus in the range of 43.91–58.01 GPa, low elastic energy (14.81–24.73 MJ/m3), and together with large plastic strain over 18.5%. High concentration of Cu ion released steadily from alloys in early 7 days, then the released concentration of Cu ion showed long-lasting and moderate. Comparing with the Ti–40Nb alloy, high antimicrobial activity was pronounced on (Ti–40Nb)–Cu alloys, and (Ti–40Nb)–Cu alloys showed more inhibitory activity against bacteria (E. coli and S. aureus) than fungi (C. albicans). Cu contents in alloys influenced the Cu ion release, which in turn affected the antimicrobial activity. As a good combination of low elastic modulus, high mechanical properties, good elastic energy, and excellent antimicrobial performance, (Ti–40Nb)–Cu alloys offer potential advantages to prevent stress shielding and exhibit an excellent antimicrobial property for hard tissue replacements.
Contributing Editor: Terry M. Tritt