Published online by Cambridge University Press: 07 March 2019
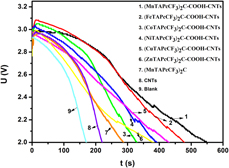
In this work, carbon nanotubes (CNTs)-templated binuclear metallophthalocyanines (MTAPcCF3)2C (M = Mn, Fe, Co, Ni, Cu, Zn) assemblies (MTAPcCF3)2C–COOH–CNTs are designed and obtained. Whereafter, the structure and morphology of target products are analyzed by many means such as infrared, X-ray diffraction, X-ray photoelectron spectroscopy, and scanning electron microscopy. The electrocatalytic performances of lithium-thionyl chloride battery catalyzed by (MTAPcCF3)2C–COOH–CNTs were carried out. The result shows that all catalysts can improve the battery performance including the discharge time and the initial voltage. The catalytic performance of (MTAPcCF3)2C–COOH–CNTs is ordered following the central metal: Mn > Fe > Ni > Co > Cu > Zn. The cell capacity catalyzed by optimal catalyst (MnTAPcCF3)2C–COOH–CNTs can expand to 28.08 mAˑh, with increase by 142.07%, and the (MnTAPcCF3)2C–COOH–CNTs can extend the discharge time to 551.6 s. Besides, the reaction mechanism is presented on the basis of cyclic voltammetry measurements.