Introduction
In 2015, the Clinical Trials Transformation Initiative (CITI) launched the Informed Consent Project to assess recommendations and observations from experts in the field of consent. The study collected data from 25 experts, representing an array of clinical trials, and concluded that the current informed consent document must be revised to be shorter, clearer, and more understandable [Reference Lorell1]. One way to address these concerns is to move the consent form from a paper document to an electronic interface, thereby enabling the use of electronic media, such as videos, interactive comprehension questions, and avatars (i.e., virtual characters), to support a customized and engaging consenting interface that could substantially enhance a research volunteer’s ability to read, retain, and comprehend key information on the study and their role as a participant [Reference Shenoy2,Reference Rothwell3].
Electronic consent (eConsent) is defined by the US Food and Drug Administration (FDA) as “the use of electronic systems and processes that may employ multiple electronic media, including text, graphics, audio, video, podcasts, passive and interactive Web sites … to convey information related to the study and to obtain and document informed consent” [4]. With the possibility for using technology to convey complicated information, research teams are considering eConsent over static paper documents for improving the overall consenting experience (e.g., increased participant comprehension, participant appeal, efficiency, and bi-directional ease of use) [Reference Flory and Emanuel5–Reference Frelich, Bosler and Gould8]. The enhanced features within eConsent provide an opportunity to facilitate recruitment and retention of participants with particular opportunities to engage underrepresented minority groups through improving understanding and the consenting experience [9]. Another driver for eConsent is the movement toward more pragmatic trials that either (1) engage directly with patients using centralized resources for consenting or (2) utilize research sites that do not typically conduct clinical trials and where paper informed consent presents a barrier to participation. eConsent could make it easier for community sites to do clinical trials and thereby increase their generalizability. With the possibilities of eConsent to improve the participant experience and extend the reach of pragmatic clinical trials, there are new considerations around regulatory compliance, remote consenting (i.e., consenting online rather than in person), proper documentation of consent, and cyber security as it pertains to protected health information. The FDA has released guidance to address these considerations with recommendations for Institutional Review Boards (IRBs), investigators and sponsors on the use of eConsent and the processes associated with obtaining informed consent for clinical investigations using various forms of digital electronic data [10].
Our eConsent framework is constructed using REDCap (Research Electronic Data Capture), a secure, web-based platform for building and managing online surveys and databases. REDCap was developed at Vanderbilt in 2004 to provide research teams a means to collect and manage research study data [Reference Harris11]. REDCap allows research teams to design data management solutions for projects using case report forms and/or participant-facing surveys. REDCap is ideal for eConsent as it allows for sharing of data within and across institutions (i.e., for multicenter trials), requires user authentication and can assign data access rights based on user role, allows for field-level data validation, and has mechanisms for ensuring data quality and integrity via an audit trail feature [Reference Harris11]. REDCap permits document storage, central data storage, and backups [Reference Harris11]. The REDCap platform, codebase, and REDCap consortium support are provided at no cost by our team at Vanderbilt with any academic, nonprofit, or government agency using a simple licensing model [12]. As of March 2020, REDCap was supporting >1 million end users at >4000 licensed REDCap Consortium partners across 137 countries.
The REDCap eConsent framework was informed by nearly 5 years of discussion with researchers, Vanderbilt IRB analysts and legal counsel, developers, and prospective participant users, in addition to conversations with stakeholders across the Clinical and Translational Science Award (CTSA) Network and the Trial Innovation Network (TIN) [Reference Zerhouni13,Reference Bernard14]. These conversations, termed “community-engaged technology development,” began at project inception and continued through pilot testing and full deployment of the framework. The process informed the specifications for eConsent portfolio content in terms of regulations and key design elements (e.g., “wet signature” – digital capture of physical signature, reviewer mode, version control management, and the look and feel of avatars and videos).
Private vendors market eConsent software (e.g., Mytrus, DatStat); however, these packages are often costly and do not translate well to the complete range of needs for researchers and their participants [15,Reference Hallinan16]. We sought to develop a generalizable REDCap-based eConsent framework to meet the following specifications:
(1) The framework must address all regulatory requirements for informed consent including 21 CFR Part 11 compliance, version control, facilitation of critical elements of consent, and be easy for IRBs, study teams, sponsors, and monitors to review.
(2) The framework and methods must be agile to accommodate tracking of multiple versions of the same consent, amendments, and multiple consent types (e.g., paediatric, adult) within the same study.
(3) The framework must be easily customizable to:
(a) the needs of the participant – that is, adaptable to “meet the participant where they are” in terms of health literacy, interest, and knowledge.
(b) the needs of a given study team, study type, or patient population.
(4) The framework should allow for collection and tracking of metrics related to a participant’s interaction with the eConsent platform (i.e., time spent watching videos, total time interacting with the consent).
(5) The framework should be scalable/transferrable and easy to adopt for REDCap Consortium partners at little or no cost.
Addressing personalization of eConsent involves tailoring to the needs of diverse prospective participants, including underrepresented minority groups. There are many recognized limitations of consent documents: length, complexity, lack of clear understanding as to what information is essential to include in a consent, inability to have one document that meets all the information and communication needs of prospective participants, and no good methods for evaluating the adequacy of informed consent from the participant perspective [Reference Hallinan17]. All of these deficits in the consent form itself make informed consent inaccessible to those with limited health literacy, defined as “the capacity to obtain, process, and understand basic information needed to make informed decisions about research participation,” which can preclude research participation [Reference Ford18–20]. Our eConsent framework was developed as part of an intervention package for the STRIDE project.
The STRIDE project is a collaboration with the University of Massachusetts Medical School (UMass), the University of Alabama at Birmingham (UAB), and Vanderbilt University Medical Center (VUMC) with the goal of developing, testing, and disseminating an integrated multilevel, health literacy targeted intervention to improve access for African Americans and Latinos in translational research. STRIDE interventions include the eConsent framework, storytelling videos, and simulation training [21,22]. Despite disparities in leading causes of death, morbidity, and disability, African Americans and Latinos are underrepresented in important research studies [Reference George, Duran and Norris23]. While not the only reason for decreased clinical trial participation, there are data that show the rate of low health literacy is higher among racial/ethnic minorities than non-Latino whites, thus providing a potential opportunity to increase minority participation in clinical trials by improving the understandability of the consent document [Reference Powell24,Reference Andrulis and Brach25].
Herein, we describe the development of our REDCap-based eConsent framework and associated features and illustrate how our eConsent process was deployed and tested for acceptability by community and research team stakeholders.
Materials and Methods
REDCap External Modules
REDCap external modules are built, deployed, and maintained by REDCap Consortium partners to extend REDCap beyond its standard distribution version. External module creators can disseminate their work for use by REDCap Consortium partners by publishing in the REDCap Repo (i.e., a centralized repository of curated External Modules that can be downloaded to local REDCap instances) [26]. As we designed the eConsent framework, our REDCap team decided that some features should be built into the main REDCap distribution codebase while other more experimental feature should be built using external modules.
Soliciting Stakeholder Feedback to Build eConsent
Community engagement studios
The Community Engagement Studio (CES) program, developed at VUMC, is a “structured program that facilitates project-specific input from community and patient stakeholders to enhance research design, implementation, and dissemination [Reference Joosten27]. This model was utilized to inform aspects of eConsent feature development.
Community Investigators
Community Investigators (CIs) are members of minority and underrepresented populations and brought diverse perspectives based on a wide range of education levels, health care, and life experiences. The CIs meet monthly as part of the STRIDE team.
Pilot Testing eConsent
Pilot testing of eConsent was conducted by UAB in conjunction with the VUMC/REDCap teams. The teams sought to seamlessly integrate the evolving eConsent protocols into the workflow of an exemplar clinical trial without increasing burden on the research teams. User testing with research staff, prospective research participants, and Principal Investigators of the exemplar study was used to determine framework acceptability for ease of use, adequacy of the material covered, level of comfort with eConsent by both research staff and prospective research participants, overall satisfaction, perceived eConsent utility, barriers to implementation, and additional training needs.
eConsent Portfolio
Based on the emphases of the STRIDE project and conversations with multiple research teams at VUMC and REDCap Consortium partners, we focused on enhancing two existing REDCap features and developing eight new features for inclusion in the REDCap eConsent portfolio. Existing features that were enhanced to support eConsent were Branching Logic – Comprehension Questions and PDF – Consent. New features are (1) video library, (2) wet signature, (3) avatar, (4) in-line descriptive popups, (5) analytics module, (6) PDF-consent document repository, (7) document vault, and (8) Part 11 Certification for the eConsent framework. All features in the eConsent portfolio are available to users at no cost except the avatar feature.
Branching logic – comprehension questions
Information is displayed sequentially to users and embedded program branching logic filters content such that participants are presented with questions or content derived from answers to previous questions. Branching logic can be used in eConsent to create comprehension questions that utilize logic to confirm participant responses, direct participants to the correct information within the document, or help study teams assess concepts which are confusing to study participants.
Feature 1: Video library
The video library presents complex procedures and research concepts that can be used to increase accessibility of study materials for persons of varying health literacy as part of the consent process. To develop the video library, we conducted a needs assessment by surveying publicly available YouTube channels associated with >60 CTSA institutions to review available educational videos. The assessment was designed to help community stakeholders provide input on desired video content and key video features needed to support eConsent. Input from CIs affiliated with the STRIDE team and community stakeholders was used to inform all aspects of video development including imagery, lighting, voiceovers, background music, representativeness of characters, and the interactions depicted between characters. Community members contributed to the videos by providing voiceovers and serving as actors. Using feedback from the needs assessment and community feedback, we developed initial content for the video library. Draft video scripts were reviewed by the VUMC IRB and Effective Health Communication Core to assess appropriateness and readability. Filming was done at VUMC. ARTMAGIC LABS (Nashville, TN) provided filming, editing, and closed captioning services. Draft videos were reviewed by community members at UAB, UMass, and VUMC. Videos are publicly available (https://www.youtube.com/channel/UCKOqWFdtVU7XsxWs2fpfvNQ) and can be easily inserted into REDCap eConsents.
Feature 2: Wet signature
We developed a field type for capturing wet signatures. REDCap allows for various field types which specify how information is displayed on the data collection instrument (i.e., how it will appear to the participant). The signature field allows users to sign a document with a mouse, stylus, or their finger. The signature is captured, stored, and appended to the eConsent as a PNG image file with a timestamp and rendered in the signed PDF documentation used to record eConsent transactions.
Feature 3: Avatar
The avatar feature provides a virtual assistant (eStaff) to guide a participant through the eConsent with voiceover instruction or clarification. We used Oddcast media software studio to create scripted avatar messaging for use with eConsent [28]. Although avatar functionality is not native in the REDCap software platform, we used REDCap’s External Module architecture to build the support infrastructure. This external module can be shared but requires adopting institutions to license and support Oddcast media software. When deployed for a project, avatars can be customized in appearance, voice, and scripts (Fig. 1). Using the avatar module requires secure, real-time “text to speech” information exchange with the Oddcast 3rd-party solution provider. To ensure data privacy, all information is encrypted during transmission, and we recommend omission of all participant identifiers in avatar-based communication scripts.
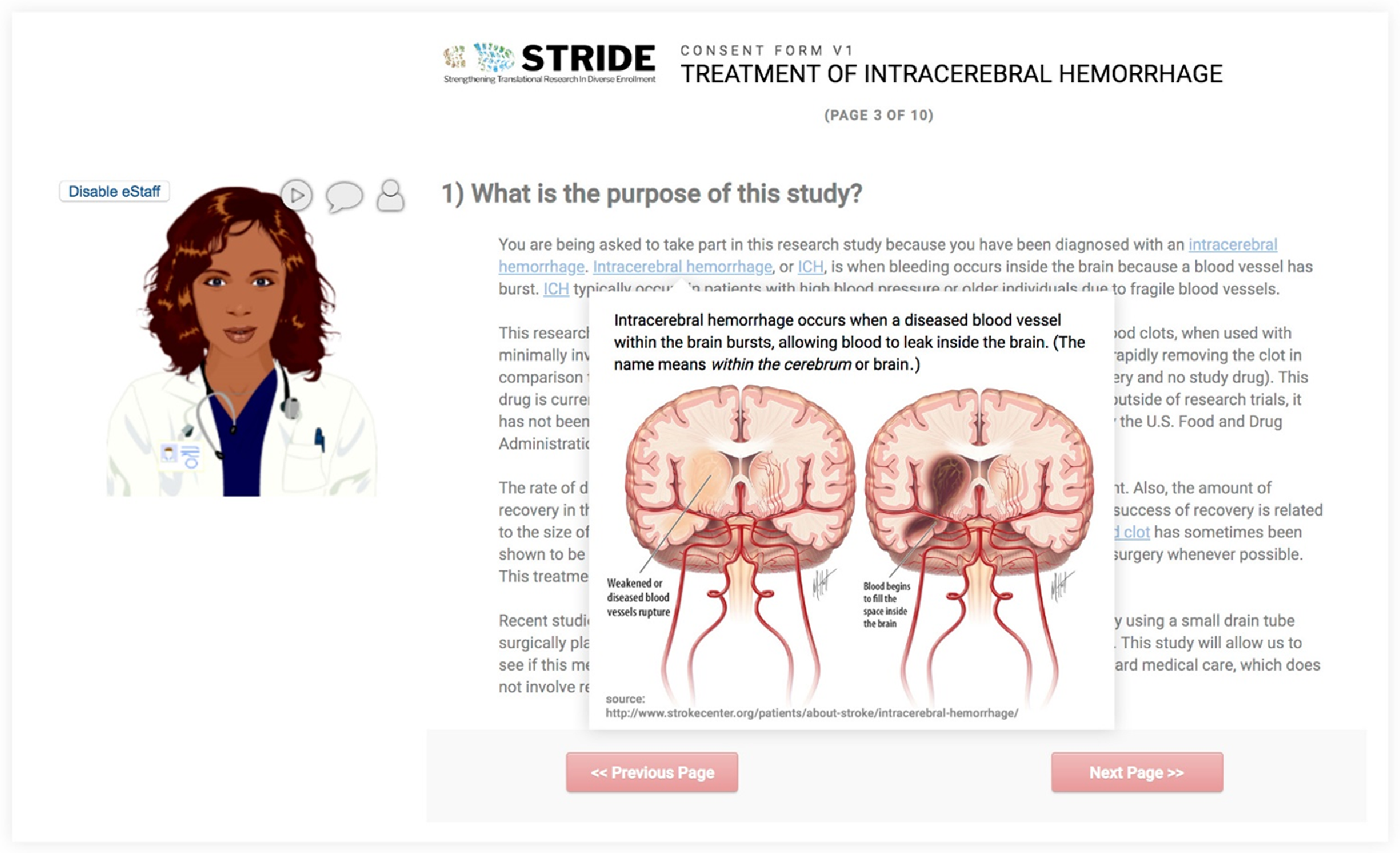
Fig. 1. The REDCap-based model of eConsent allows for the incorporation of avatars, virtual assistants that can be self-selected by participants. Additional features include the use of “hover and click” in-line descriptive popups that allow participants the option to obtain more information (e.g., pronunciation, images, and definitions) about key words in the consent.
Feature 4: In-line descriptive popup
This external module allows researchers to deploy supplemental information (e.g., text definitions, pronunciations, explanatory images) for review by prospective research participants. Supplemental information is linked to individual words or phrases and highlighted within an eConsent document so that when a prospective participant hovers over a linked word, supplemental information is displayed in a popup window. This feature allows prospective participants to decide how much detail they want from the eConsent document (Fig. 1).
Feature 5: Analytics module
This external module functions in the background of eConsent survey mode to capture metrics about how a participant interacts with features of the survey, such as videos, avatars, and in-line descriptive popups.
eConsent Transaction and PDF Rendering
PDF-consent
When a REDCap survey or eConsent is completed, participants can have a PDF copy of their consent printed or emailed to them. The “PDF-consent” tool generates a streamlined PDF that removes unselected choices and irrelevant branching logic content (e.g., material not shown to participant because it was not relevant).
Feature 6: PDF-consent document repository (i.e., “auto-archiver”)
The “Auto-Archiver + eConsent framework” option adds two things to the typical REDCap survey process. (1) Before a participant completes the survey, a certification page is added to the end of the survey that displays an in-line PDF copy of their responses in which they are asked to confirm that all information in the document is correct. The consent documentation will not be considered complete until the participant fulfills the certification step. (2) Upon completion of the eConsent, a static copy of their responses in the form of a consent-specific PDF will be stored in the project’s File Repository.
Feature 7: Document vault
We established within the REDCap eConsent framework module the ability for institutions to specify settings for a local secure file transfer protocol (sFTP) site. The rationale behind this decision was (1) to allow redundant storage outside the REDCap system and (2) to create a small digital footprint for 21 CFR Part 11 (or Part 11 herein) validation (transaction begins at a point where participant clicks approval on the rendered PDF-consent document and ends with storage in the restricted access sFTP system).
Feature 8: Part 11 certification for eConsent
21 CFR Part 11 refers to the Code of Federal Regulations, established by the FDA to govern the criteria under which electronic records and signatures can be considered secure and trustworthy (i.e., equivalent in fidelity to paper records) [29]. Software cannot be Part 11 compliant alone – procedural controls are also required. VUMC has verified and documented its software and required procedural controls with the validation of the REDCap eConsent framework module. Validation of the VUMC eConsent framework was conducted by JAF Consulting, Inc. (Philadelphia, PA). The methods and procedural controls for establishing a Part 11 compliant eConsent framework have been documented and shared with REDCap Consortium partners interested in obtaining official certification. Those sites seeking to obtain certification have the option of utilizing the VUMC shared scripts, controls and validation SOPs, or hiring an independent Part 11 consultant to complete the validation process.
Community-Engaged Technology Development
CESs and CIs were used to solicit community feedback during the development of our eConsent framework and feature set. CES allowed our research team to obtain feedback from stakeholder groups who were not familiar with eConsent or the goals of STRIDE. Specific groups engaged were study coordinators, regulatory teams, and community members/patients from underrepresented minority groups. In contrast, the CIs are embedded within each STRIDE site study team at the operations level and are not research or project “naïve” as is the case with CES participants. African American and Latino community representatives are integral team members who have drawn upon the wisdom of their respective communities to inform, at the earliest stages of the project, eConsent feature development, pilot testing, implementation, and dissemination. Community representatives were identified through prior participation in CESs and engagement with community-based organizations and local advocacy work. Vanderbilt community representatives are actively engaged in prison reform initiatives, community development boards, and financial coaching in underserved populations.
Results
Community Engagement Studios
We conducted nine CES to solicit feedback from community members and research assistants before and during the building and implementation of the eConsent framework, video library, and associated features. Table 1 summarizes the participant characteristics, focus, and recommendations of the Studios conducted at each of the three STRIDE sites.
Table 1. STRIDE community engagement studios – key recommendations
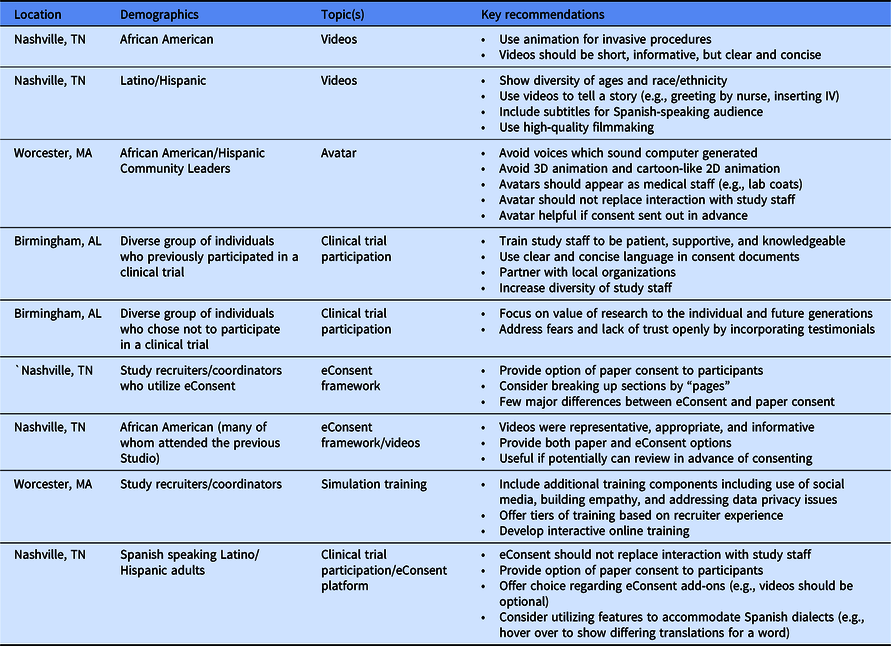
After reviewing sample videos, Studio attendees emphasized the importance of representing minority populations and encouraged creating vignettes by depicting supportive and helpful interactions with medical staff (e.g., greeting patients) and expressed a preference for use of animation to depict invasive procedures (e.g., lumbar puncture). Studio attendees noted that avatars should not replace interactions with study staff. In separate Studios, community members and study recruiters reviewed the completed eConsent framework. Recruiters recommended providing paper consent as an option for participants who may be uncomfortable with or who have difficulty accessing technology. Community members echoed the importance of offering a paper option and suggested sending the consent in advance of a meeting with the study coordinator. With a few individual preferences, the feedback from the community members regarding elements of eConsent and the video library was notably consistent across gender, age (19–84 years), and educational background (high school to post-graduate degree).
Community Investigators
The STRIDE team has engaged seven CIs. The CIs provided feedback on the consent experience and individual elements of the consent including videos, avatars, and consent language. Their involvement informed the tailoring of features and documents to meet the varying levels of health literacy for prospective research participants. The input of the CIs helped to develop many of our eConsent features (e.g., avatars and actors/interactions in the videos) such that the depictions of people are more representative of the diversity that researchers hope to achieve within research studies.
Video Library
Community feedback was used by our team to develop storyboards and voiceover scripts for eight procedures and/or research themes as topics for 10 videos that we created for the library (Table 2). The videos are short (1–4 min in length) and represent specific procedures with a voiceover explanation to describe each step of the process. In an effort to improve the accessibility of the videos to those of varying health literacy, the scripts were reviewed by the VUMC Effective Health Communication Core to reduce word count/sentence count and words per sentence. Scripts were generally written at a Flesch-Kincaid Grade Level of 6; however, the need to define acronyms involving medical terms such as magnetic resonance imaging and computed tomography resulted in final scripts at a 7–8th grade reading level.
Table 2. Common research procedures and informed consent elements in the eConsent video library

** Denotes videos developed in collaboration with the Recruitment Innovation Center (RIC).
Implementation of eConsent
We are implementing and evaluating eConsent interventions as part of STRIDE studies and will report eConsent metrics and feedback in the context of individual study manuscripts. The eConsent feature set and framework were released to the global REDCap Consortium in March 2018. As of March 2020, >200 institutions have enabled the eConsent framework with approximately 140,000 eConsent transactions [Reference Harris11].
Discussion
General Workflow for REDCap-Based eConsent
Based on pilot testing at other sites, we created a generalizable model for development and review of eConsent: (1) investigative team prepares a participant-facing eConsent survey instrument in REDCap and submits the document for IRB review (Fig. 2); (2) submission should include a static copy of the REDCap survey (an auto-generated, nondynamic PDF), links, transcripts for any embedded multimedia (e.g., videos), and a link to the REDCap eConsent survey for IRB review and approval, and (3) commitment from investigators to follow traditional procedures for consent documentation best practices and accountability. Investigators are responsible for ensuring that the version signed by participants matches the version approved by the IRB at the time of signature. Investigators must maintain version control of eConsents should they need to update/amend the consent document during the study. The REDCap eConsent framework automatically saves all consent documents to a central location so that researchers can easily sort, find, and review previously signed consent documents based on consent version, individual participant, and date.

Fig. 2. Workflow for eConsent development, IRB approval, and document management.
Ethical considerations were identified during piloting that relate to the use of the analytics module which captures data on how a user interacts with the platform. Specifically, community members and IRB analysts voiced concerns about a participant’s right to know that their eConsent behavior is being recorded. To address these concerns, we developed standardized language to be included at the beginning of any eConsent in which this module will be enabled, “We would like to improve the consent experience for our research volunteers. Today, we will be collecting information about how you have used this electronic consent form”. This language was included as part of all STRIDE studies, vetted with community members who were familiar with the project and the specific analytics module, and the STRIDE site IRBs.
STRIDE Pilot Testing
Refinements were made to the eConsent framework and workflow based on pilot testing and STRIDE team brainstorming. Refinements included:
(1) Reviewer mode – a REDCap survey can be enabled for reviewer mode so that a member of the study team can summarize the eConsent document with a prospective participant without the need to complete required fields or having avatars enabled. This mirrors the workflow using a paper consent whereby a study team member provides a summary of the consent document to the participant before allowing them time to review.
(2) Analytics dashboard – a dashboard was created to help visualize data captured by the analytics external module. The dashboard provides participant-level data detailing time spent on each page of the consent, cumulative time of the consent process, and time spent engaged with videos, avatars, or in-line descriptive pop ups.
(3) eConsent preview – UAB piloted eConsent “previewing” in which a survey link is sent to a participant in advance of being officially consented in the clinic. In the preview mode, the wet signature field is removed allowing participants to read through the document, view videos, and interact with the avatars without signing.
Lessons Learned and Challenges of the eConsent Framework
Feedback from the STRIDE sites has helped identify challenges related to use of eConsent in the real world and initiation of a series of best practices. Users are encouraged to engage their local REDCap teams early in their utilization of the eConsent framework to define project needs and timelines for implementation of specific features. To address regulatory-based challenges include balancing the flexibility needed by study teams with the security and limitations of the system imposed by the document vault and Part 11 compliance. Effectively, once a participant signs, confirms, and submits their eConsent, the record is “locked” in the virtual document vault and further changes are not permitted to the document. This design, intended to maintain the fidelity of the record, has posed some challenges to workflow, as “common sense” edits, cannot be saved/overwritten in the vault. Examples include going back into a record to correct a typographical error without having to initiate a new consent. We continue to develop REDCap infrastructure to mitigate this issue with the goal of making it easy to “do the right thing” for investigative teams, without creating unnecessary burden.
Future Directions and Recommendations for Implementation of a Similar System
Part 11 compliance
As part of the STRIDE project, UAB and UMass are working with the VUMC REDCap team to update their local REDCap instances, implement the new features, and pursue Part 11 compliance certification for their eConsent frameworks.
Remote consenting and telemedicine
There is increased interest in the use of telemedicine, and by extension, remote consenting to help reach prospective patients/participants in rural healthcare settings, as well as to help address issues of health disparities, as rural communities are often underrepresented in research. Current evidence suggests that telemedicine, which can include video conferencing, web-based materials, and other media, under certain circumstances, can provide care equal to that of in-person interactions, with potentially lower cost [Reference McConnochie30,31]. Additionally, early adopters of remote consent via multimedia platforms have found that utilization of such platforms can streamline the consent process, are appealing to prospective participants as users are more likely to opt for the remote option of consent (if available), and users have demonstrated equal satisfaction with in person vs remote consent options [Reference Schallhorn32,Reference Haussen33].
Toward this end, some STRIDE exemplar studies utilized eConsent with a remote consenting component. For example, the UAB STRIDE exemplar study will send the eConsent for review, but not signature, by the participant prior to consenting in person at a clinic. Early results using remote eConsent with the Vanderbilt STRIDE exemplar study suggest that utilization of eConsent may increase the participation of minority pregnant women in prospective research. Researchers concluded that eConsent was effective for consenting women with low health literacy [Reference Phillippi34]. Further work over the course of the STRIDE project and more detailed data capture of participant interactions with the eConsent framework will assess the impact of the framework on recruitment and retention of minority participants in research studies and help to inform best practices for utilizing the framework in remote consenting.
Continuing the consent conversation
Consent is not just a one-time event but rather a continual process throughout the study. The eConsent framework provides a mechanism by which study teams can continue the consent conversation. Study teams may consider sending their eConsent public survey link in preview mode (i.e., no signature block but contains all other features) along with an appointment reminder and utilize this as a mechanism to revisit the consent and keep the conversation going with their research participants. This may help improve the accessibility of the study material as literature suggests that the informed consent conversation often uses easier words and is shorter than the consent document [Reference Koyfman35].
Managing eConsent for multicenter trials and sIRB
VUMC is working with the TIN on several multicenter studies to begin piloting processes and methods around using eConsent with multicenter studies and single IRB (sIRB) review [36]. The two main areas of focus have been regulatory (IRB review process) and consent management/housing of consents:
Regulatory – sIRB review integrates seamlessly with the use of eConsent as multicenter studies often utilize a 2-part consent in which Part 1 represents study-specific information that is universal across all sites (reviewed by the IRB of record), and Part 2 is site specific (reviewed by the respective relying IRBs along with other relevant local context). The documentation described above (see section STRIDE Pilot Testing) provides a starting point for review by both the IRB of record and relying sites.
Consent management – There are two models by which eConsents can be managed for multicenter studies: (1) the Data Coordinating Center (DCC) creates and manages a single eConsent repository in REDCap where individual site personnel may access information for only their sites or (2) each site maintains, builds, and stores their own eConsent documents locally and shares data directly with the DCC. While the setup for the DCC is greater if it manages and stores (on a local server) all of the eConsents for the study, the advantage is that version control and document management becomes streamlined, and for Part 11 applicable studies, only the site managing the consents needs to have a Part 11 validated instance of REDCap.
Video library
As VUMC (and other sites) continues to develop educational videos, we recognize an opportunity to develop a video library functionality within REDCap to make videos easily “discoverable” for users. Current work is seeking to embed a video library within REDCap that includes educational videos reviewed and approved by the VUMC IRB, with potential enhancements to allow other local IRBs to review and indicate their approvals. Ideally, users would be able to filter the video library based on procedure/theme and determine which institutions have registered their approval of a specific video. While we acknowledge that approval by one IRB will not ensure approval of videos by other IRBs and that videos must still be reviewed on a study by study basis with appropriate context, the video library may help facilitate eConsent review and approval in the current era of sIRBs.
eConsent training
The collaborative nature of REDCap users lends itself to rapid growth in this space as developers and researchers continue to test the framework with new use cases and advance methodologies to meet the needs of their stakeholders [Reference Harris37]. Despite the strengths of the REDCap platform, implementation and utilization of various features are only as good as the training and support available for these tools. There are a variety of resources to support building and designing eConsent projects in REDCap including tutorials that detail step-by-step project build-out, webinars to describe new eConsent features, and community involvement in the development process, as well as guidance available through the TIN to support studies seeking to use eConsent [36,Reference Harris38–40].
Conclusion
The eConsent features described herein are being circulated within the REDCap Consortium and disseminated using webinars and “Toolbox” sharing mechanisms via STRIDE online materials and the TIN Recruitment Innovation Center [12,Reference Bernard14]. We continue to refine features based on continued community-engaged technology development methodologies. We are combining the STRIDE project experience with anecdotal feedback from our larger REDCap worldwide research community that adopted the eConsent framework to define and prioritize new “wish-list” features for development. These include embedding a video library within REDCap, improving avatar functionality for voiceovers (including non-English languages), and possibly integrating eConsent with electronic health record systems. We look forward to using the implementation of eConsent projects to gather evidence to support our hypothesis for STRIDE that the eConsent framework provides an opportunity to improve access of underrepresented minority groups for translational research by facilitating engagement with the consent process.
Acknowledgments
The authors extend their sincere thanks to Jackie Sims, Daniel Cruz, and the Community Investigators at UAB and UMass for their input and guidance. This project was supported by awards: STRIDE (5 U01 TR001812), UMass CTSA (5UL1 TR001453-04), UAB CTSA (5UL1 TR001417-04), VUMC CTSA (UL1 TR002243), and The Recruitment Innovation Center – RIC (5U24 TR001579-02) from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Disclosure
The authors declare no conflicts of interest.






