Introduction
South Asians (SAs), individuals from India, Pakistan, Nepal, Bangladesh, and Sri Lanka, comprise nearly a quarter of the global population with over 25 million residing in diaspora countries. There are approximately 5 million residents of SA origin in the USA [1]. SAs are the second largest Asian subgroup and are the second fastest growing ethnic group in the USA after Latinos [2]. SAs have a unique phenotype with a high prevalence of early onset cardiovascular disease (CVD)[Reference Joshi3,Reference Zaman and Bhopal4] where conventional risk factors (age, sex, smoking, obesity, diabetes, cholesterol, and hypertension) do not fully explain this heightened disease risk [Reference Joshi3,Reference McKeigue, Miller and Marmot5]. To fill this gap in knowledge, the National Heart, Lung, and Blood Institute supported the creation of the Mediators of Atherosclerosis in South Asians Living in America (MASALA) prospective cohort study to investigate the prevalence and risk factors associated with subclinical atherosclerosis at baseline and incident CVD events among a community-based sample of SAs in the USA aged 40–84 years without known CVD [Reference Kanaya6].
We completed the second clinical exam of the MASALA study and enrolled a new wave of participants by March 2018. We describe the methods employed for participant follow-up/retention and the new wave of recruitment, compare characteristics of the new enrollees with the original cohort and the participants who did not follow-up, and discuss the barriers and facilitators for retention and recruitment of SA participants in a longitudinal cohort.
Materials and Methods
Our original study eligibility and recruitment methods have been described previously [Reference Kanaya6]. The MASALA study was conducted at two academic institutions, the University of California San Francisco (UCSF) and Northwestern University (NU). A total of 906 community-dwelling participants from the San Francisco Bay area and the greater Chicago area were recruited at baseline (2010–2013). A new wave of participants was recruited from March 2017 to March 2018 (Fig. 1). The study was approved by the institutional review boards at UCSF and NU.
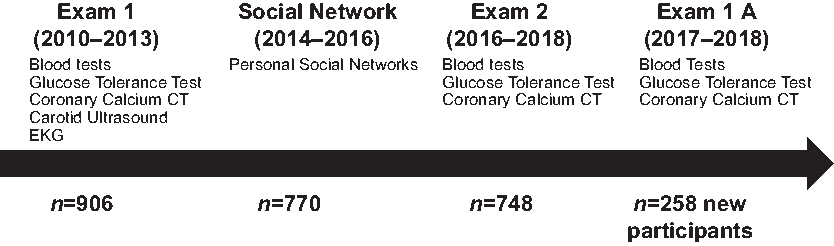
Fig. 1. Flow diagram for the MASALA study exams. EKG = electrocardiogram; CT = computed tomography.
Briefly, to be eligible for the MASALA study, participants had to (1) self-identify as SA; (2) be between 40 and 84 years of age; and (3) have the ability to speak and/or read English, Hindi, or Urdu. The remaining eligibility criteria were identical to the Multi-Ethnic Study of Atherosclerosis (MESA) [Reference Bild7] and excluded those who had heart attack, stroke or transient ischemic attack, heart failure, angina, use of nitroglycerin, a history of CVD procedures or any surgery on the heart or arteries, current atrial fibrillation, or active treatment for cancer. Individuals with life expectancy <5 years, those with impaired cognitive ability, or those who planned to move out of the study region in the next 5 years were also excluded. For our second wave of participant recruitment, we used a higher age criterion of 50–84 years, so that new participants would be similar in age to those already enrolled in the cohort. Written informed consent was obtained from all participants at the start of each exam visit. Consent forms were translated into Hindi and Urdu and trained bilingual research coordinators obtained consent.
Retention Methods
After the baseline exam visit, each participant received an annual study newsletter, a birthday card, and a holiday card (Diwali, Eid, or Christmas, as appropriate based on their religious affiliation). We organized community forums to disseminate baseline MASALA study results to participants/community members and held several health and wellness events for participants. We contacted each participant annually for a brief follow-up by phone [Reference Kanaya6] and/or by email [Reference Kandula8] to ascertain general health status and asked about any new health diagnoses, procedures, hospitalizations, or intervening CVD events.
Ancillary and Second Clinical Exam
In September 2014, we began a social networks ancillary study inviting all surviving MASALA study participants for a 2-hour interview with physical exam measurements (Fig. 1) [Reference Kandula9]. The data collection for the social networks study did not include any blood draws or imaging procedures, and most visits were conducted in convenient community locations or in the participant’s home. Participant remuneration for this brief visit was $25.
In September 2015, participants were contacted by email and also mailed invitational letters to return for a second clinical exam which included fasting blood tests, an oral glucose tolerance test (OGTT, for those without known diabetes), anthropometry, seated blood pressure, and a repeat non-contrast cardiac computed tomography (CT) scan for coronary artery calcium (total exam time of 2–3 hours). Participant remuneration for Exam 2 was $25 to help to pay for transportation and parking costs. We first contacted participants who had not completed the social networks ancillary study and combined both Exam 2 and social networks data collection together in a 3–4 hour study visit. Participants who completed both study visits together received $50 remuneration.
Assessing and Addressing Participant Burden for Exam 2
In mid-2016, we informally asked participants about the barriers and facilitators that would make it easier for them to attend and complete Exam 2. In late 2016, we started employing new approaches to engage and retain participants to help overcome the main barriers of distance, time, and transportation and also to increase their understanding and engagement in the study. We offered the new retention strategies as several options to participants who had not responded to our initial invitation (by mail and phone). We report the additional cost for each of these retention approaches exclusive of the time and effort by our research study staff.
To lessen participant time and travel burden, we opened two new suburban clinical sites (both through Northwestern Medicine, in Winfield and Glenview, Illinois) approximately 35 miles east and 15 miles north of Chicago, respectively. These locations were much closer to participants’ homes. Participants who reported being too frail to take public transportation or drive to the clinical exam were offered a cab or shared ride reimbursement from their homes, or a MASALA staff member to travel with him/her to the clinic site, or an abbreviated home visit which would not include an OGTT or CT scan. We also provided Saturday appointments at both the UCSF and NU sites, offered raffle prizes to exam completers, and had the principal investigator (PI) at each site perform telephone outreach. Participants who had moved out of the geographic area of the clinical site were offered an airline or train ticket, a one-night hotel stay, meals, and taxi transfers to attend the clinical exam. We also conducted monthly community workshops in different geographic locations at each clinical site to disseminate MASALA study findings and provided education about wellness topics such as mindfulness, stress reduction, yoga, healthy SA cooking, and resistance exercise training. At these community events, the PI and study staff would briefly describe study progress and emphasize the importance of longitudinal follow-up and completing clinical Exam 2.
There were two additional retention strategies employed by the NU site in mid-2017. Participants were invited to bring in their spouse for a limited exam with free fasting blood test and other physical exam measurements. The NU site also increased remuneration to $150 during the last 2 months of the study period to help overcome time and transportation barriers.
Recruitment of 2nd Wave of Participants
Our goal was to recruit approximately 250 new participants into the MASALA study to increase the overall size of the cohort. To create internal diversity in our study population, we purposefully targeted recruitment to include more SA women and include groups that were relatively under-represented in our original cohort (those born in SA countries other than India) and those with lower socioeconomic status.
Our two field sites used three primary methods of recruitment, allowing each site to use the strategies that would be most effective based on their experience with working with the SA community and organizations locally. First, we engaged community organizations that serve SAs and study staff presented informational sessions about the goals and findings to date of the MASALA study. At UCSF, we engaged community leaders in the Pakistani, Bangladeshi, and general Muslim communities and gave talks about SA heart disease and the goals of the MASALA study in different community settings (Pakistani American Association, South Bay Islamic Association, Muslim senior support network, and a Bangladeshi community group). At these community informational seminars, we collected contact information from attendees who were interested in learning more about the study and being screened for eligibility. As a distinct method of community outreach, the NU site also hired a community recruiter who was a staff at an SA community organization; this individual recruited individuals into the study through face-to-face recruitment at local religious and community organizations. The community recruiter used fliers and explained the study to people. The recruiter provided the contact information of interested individuals to Northwestern field center staff who followed up with a telephone call in 1 week.
Second, we used a chain referral approach and asked current study participants to recommend up to three unrelated SA contacts (with no monetary incentive for the referral). This chain referral approach utilized a snowball sampling method and is commonly used in studies of harder to reach populations [Reference Bagheri and Saadati10]. Once the contact information was obtained for these referred individuals, study staff screened the person for eligibility by phone and determined whether the individual already had a household or family member in the study as an additional exclusion criterion. Newly recruited participants were also asked to refer up to three individuals into the study after completing their exam visit to create separate waves of recruitment that extended beyond the current participants.
Lastly, the NU site used the electronic health record (EHR) at Northwestern Medicine to generate lists of patients from the healthcare system who may be potentially eligible for the MASALA study. Individuals of SA origin were identified using an SA surname list [Reference Lauderdale and Kestenbaum11]. After receiving permission to contact the patient from their primary care provider (PCP), these letters were personalized and were signed by the MASALA study PI. We mailed invitational letters and brochures to random batches of these patients and called them within 2 weeks to determine their eligibility and interest in participating in the study. Because these individuals were already patients of the healthcare system, we expected fewer barriers to participation in the clinical exams and better access to health records for ascertainment of CVD events.
Statistical Analyses
We compared participant characteristics between those who completed Exam 2 and the newly recruited individuals, and also compared Exam 2 participants with non-responders using chi-squared tests and t-tests for these comparisons. We used SAS, version 9.3 (SAS Institute) for our analyses.
Results
After a median 4.8 years of follow-up, a total of 749 (83%) of the surviving MASALA cohort (n = 900) participated in Exam 2 with higher retention at the UCSF site compared to NU (88% vs 76%). Another 258 participants were recruited in the second enrollment wave (called Exam 1A), with 55% recruited from the greater Chicago area and 45% from the San Francisco Bay area. Table 1 shows the demographic characteristics of the MASALA study cohort at each exam and the new wave of enrollees. The new enrollees in Exam 1A were older than Exam 2 participants, had lived in the USA for fewer years, were more likely to be from Pakistan and Sri Lanka, and had lower educational attainment compared to existing participants. Fig. 2 compares the participants who completed Exam 2 with the surviving cohort participants who did not return for Exam 2. Compared to participants who completed Exam 2, non-responders were more likely to be women, Pakistanis, Muslim, and have lower educational attainment.
Table 1. Characteristics of Mediators of Atherosclerosis in South Asians Living in America (MASALA) participants at each clinical exam

Values represent n (%) or mean ± SD.
Age and years lived in the USA are estimated with a censoring date of March 1, 2018.
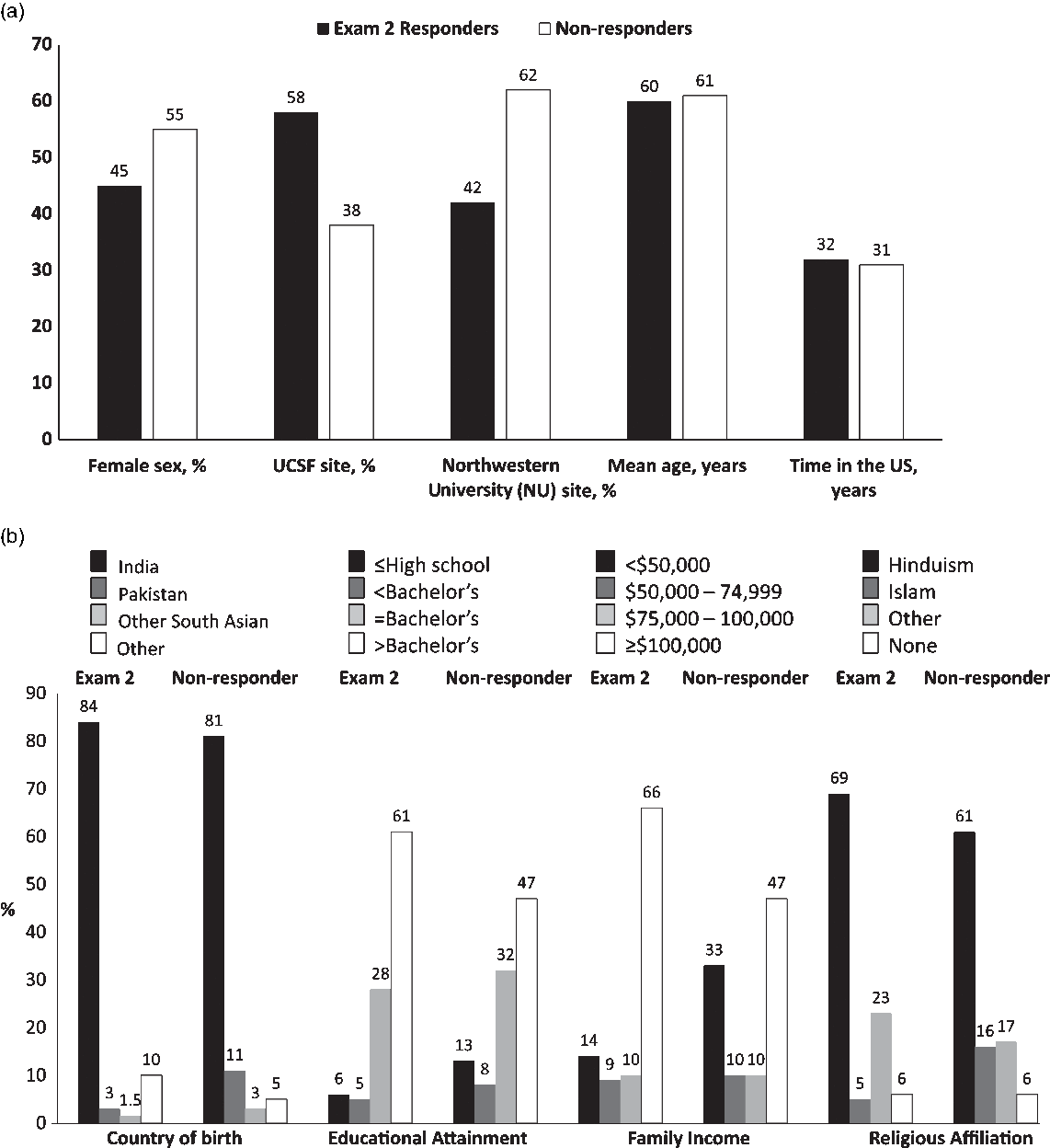
Fig. 2. Characteristics of Mediators of Atherosclerosis in South Asians Living in America (MASALA) participants who completed Exam 2 compared to non-responders.
To develop relevant retention approaches, we contacted participants by phone and with emails to assess barriers for follow-up and found that the clinic location, travel logistics, and overall time for the study visit were the biggest barriers to continued participation. The clinical exam protocol included a fasting blood draw, a 2-hour OGTT, a cardiac CT scan, along with physical exam measurements and questionnaires which took on average 3–4 hours to complete. While some participants felt that these tests were incentives because they are not routinely provided as part of medical care, some individuals feared repeating the CT scan in the second exam due to possible harm from radiation, and several reported discomfort with the oral glucose solution for the OGTT. Participants were able to opt out of any tests or procedures that they did not wish to perform (91 did not do the OGTT and 47 participants did not complete the CT scan during Exam 2, which includes the 29 people who did home-based visits).
Several of our newer retention approaches were helpful for Exam 2 completion (Table 2). The NU field site opened two new community hospital locations (in Winfield and Glenview, Illinois) that approximately half of all of the participants (n = 150) preferred for their study exam than the downtown Northwestern University Medical Center. The overall cost associated with training and certifying the nursing and laboratory staff on the study protocol was approximately $2800 at each of the two hospital locations. Another approach that was helpful in retaining participants (n = 51) was to provide reimbursement for a taxi or shared ride cost to travel to the clinical exam. The average cost of this transportation expense was $132 at UCSF and $85 at NU. Another useful retention method was conducting abbreviated exams in the participant’s home. With transportation cost for the staff member and phlebotomist to draw the blood samples and transport them to the lab and clinical site, the average additional cost for the home visit was $140 per participant. As a novel retention strategy, the NU site allowed a participant to bring in a family member (most commonly a spouse) who would receive a limited free clinical exam with fasting laboratory tests (for an additional cost of $41 per participant), which resulted in approximately 29 retained participants. Travel reimbursement for people who had moved away from the geographic area helped to retain eight respondents (average cost of $600), and higher remuneration for exam completion was used by six respondents.
Table 2. Participants seen using newer retention methods
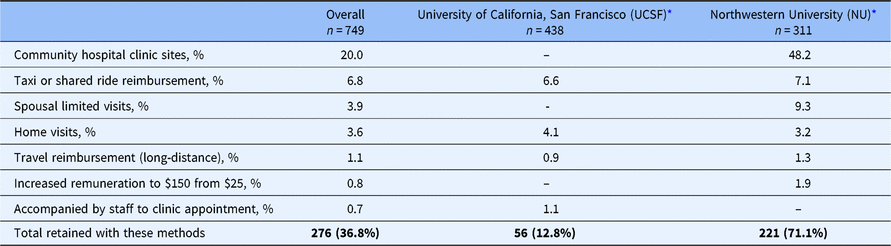
* Three participants moved from the Chicago area to the San Francisco Bay area between Exam 1 and Exam 2 and were seen at the UCSF field site.
Recruitment yield for our new wave of enrollment is shown in Fig. 3. We identified a total of 1067 individuals (317 at UCSF and 750 at NU) over the course of 1 year (March 2017–Feb 2018) comprising our total recruitment pool. Approximately 55% of the recruitment pool at NU was derived from the EHRs at NU medical center, but the participants who enrolled using this approach was lowest; only 5% of those who were mailed invitational letters enrolled in the study. The EHR recruitment cost was approximately $5000 or $227 per enrolled participant. The community outreach had a higher yield and was similar at both sites with about 28–30% enrollment of those who provided contact information. NU received more contacts through the community recruiter than by direct community outreach events held by the study staff. However, the proportion of eligible and enrolled participants was higher with the field center staff’s community outreach than by using a community recruiter (57% vs 26%). The additional cost for the community recruiter method was $4440 or approximately $78 per enrolled participant. The participant chain referral method had the highest yield overall with no additional cost, with 42% at UCSF and 51% at NU enrollment from this approach. However, only 9% of participants from NU and 18% of participants from UCSF referred their friends, and the average number of referrals was 1.6 and 1.9, respectively. A total of 89 (8.3%) individuals who were screened for the study were found to be ineligible, and the primary reason for ineligibility overall was existing CVD. Of those who were eligible for participation, 50 (4.7%) were not interested in enrollment. A total of 258 SAs, or 82% of those who were found to be eligible, enrolled in Exam 1A, which was 24% of the overall recruitment pool.

Fig. 3. Recruitment approaches for the new wave of participants in Mediators of Atherosclerosis in South Asians Living in America (MASALA), 2017–2018.
Discussion
SAs are underrepresented in health research, and the challenge of recruiting and retaining SAs into clinical research studies remains a key issue. In this first longitudinal cohort study of SAs in the USA, the overall retention after approximately 5 years of follow-up was 83%. We found that older participants, women, Muslims, and individuals from lower socioeconomic strata were less likely to follow-up during the second clinical exam. Providing study exams at community hospital sites closer to where participants lived, offering cab or shared ride reimbursement to and from the clinical site, allowing limited laboratory testing for a family member, and conducting home visits for elderly or frail participants were the most effective methods that bolstered study retention. For our new wave of recruitment, we preferentially targeted the sociodemographic groups with poor retention using new recruitment and engagement methods to broaden the overall generalizability of our cohort. We found that participant referrals and active community engagement by study staff and a community recruiter were the best methods for recruiting eligible participants, whereas using EHRs data to mail invitational letters had a very low yield.
Very few studies have long-term follow-up of SA (>3 years) and only a handful of studies have reported or analyzed their retention methods even for shorter-term studies [Reference Waheed12–Reference Islam14]. Some strategies to aid retention that have been suggested for intervention studies in SA included providing meaningful incentives (including free transportation and childcare), hiring culturally concordant community health workers, and having flexibility in scheduling study visits [Reference Waheed12–Reference Islam14]. Retention strategies that have been successful in other race/ethnic groups including African Americans and other lower-income populations in the USA include incentives, personal approach, utilizing a dedicated phone line, project identity and logos, participant convenience, and repeated contact with participants [Reference Nicholson15,Reference Nicholson, Schwirian and Groner16]. As a result of informal participant feedback about the barriers for continued participation, we implemented several new strategies including opening new community hospital locations for the clinical exam, providing local and long-distance travel reimbursements, and home visits. While several of these strategies required more resources, they greatly reduced participant burden and improved study retention. An innovative strategy that has not been described previously was the use of a limited exam with laboratory tests for the participant’s spouse at the NU (Chicago) site. This strategy may be important for retention and engagement of SA populations where family plays an important role in health-related decisions. We found that women, in particular, brought their spouses to these limited visits. This strategy was used only at the NU site, where retention of women was more challenging than at the UCSF site, highlighting the need for strategies that may vary across SA groups and those living in different regions.
In our second wave of recruitment for the MASALA study, we employed three different strategies that were not used in our original cohort recruitment during 2010–2013 using recommendations from the recent reviews [Reference Quay17, Reference Mukherjea18]. Based on our participant demographic characteristics and early study retention results, we created three new approaches to reach women, older individuals, non-Indian SA, and individuals with lower educational attainment. We found that the community outreach and community recruiter approach were helpful in recruiting these more vulnerable populations. We learned that it was important to spend time at community events and provide concrete examples of how research could benefit the SA community and why longitudinal follow-up is important. Effective strategies are needed to increase the SA community’s trust and understanding of scientific research. Future studies should consider how to engage SA community members throughout the research study, conduct research that includes community priorities, and include community members as part of the study team [Reference Selker and Wilkins19]. Recruitment and retention of diverse populations are necessary to increase the impact and generalizability of scientific research, and community-engagement strategies are increasingly being incorporated into study design [Reference Nicholson, Schwirian and Groner16, Reference Butler20].
We also used chain referral with a snowball sampling method and asked our participants to refer up to three of their SA friends or acquaintances who may be eligible and interested in study participation. We hypothesized that this method would be effective as it has been used in several studies of ethnic minority populations in the USA. However, we found that relatively few referrals were made by the NU participants in MASALA compared to the UCSF participants. But this was the most effective method with highest yield for enrolling new participants from both sites. Although chain referral methods are commonly used in studies of harder to reach groups, including immigrants, this method is prone to sampling biases because individuals have an unequal probability of selection. Another chain referral method is respondent-driven sampling [Reference Heckathorn21]; however, this approach would have required more resources for tracking and incentive payouts for referrals [Reference Platt, Luthra and Frere-Smith22].
We used the EHR data of Northwestern Medicine to identify and invite SAs who were patients at and familiar with the healthcare setting for study screening. This strategy was expensive and had low efficacy in enrolling new participants. One weakness of our EHR approach was that we did not engage health providers heavily in our recruitment; PCPs were informed that their patients would be receiving a recruitment letter and that they could opt out. Other studies suggest that greater PCP involvement may lead to better yield when using EHR recruitment [Reference Quay17].
Several prior studies have examined strategies for improving recruitment in SA communities [Reference Waheed12, Reference Quay17, Reference Mukherjea18, Reference Douglas23, Reference Mahmood, Afshar and Tang24]. A recent scoping review by Quay and colleagues which included 15 articles that discussed barriers and facilitators for recruitment of SA found that the main facilitators were perceptions for improved treatment and health for themselves, altruistic beliefs about contributing to general health knowledge, and a sense of obligation to their healthcare providers [Reference Quay17]. Major barriers to recruitment included disinterest or lack of feeling of belonging, conflicts, education- or training-related deficits, logistical or opportunity costs, fears and inhibitions, and research-related barriers [Reference Quay17]. Recommended recruitment strategies included language and culturally driven methods, communication and engagement strategies, logistical changes and accommodations, policy and study design measures, and incentives [Reference Quay17]. Another recent paper by Mukherjea et al. described four different research studies of SAs in the USA and discussed facilitators and barriers of recruitment for each [Reference Mukherjea18]. Some promising recruitment strategies proposed include active recruitment with community talks and follow-up from study staff, providing appropriate incentives, engaging cultural research brokers, and having trusted sources of information [Reference Mukherjea18].
Several systematic and qualitative reviews have examined the barriers and facilitators for race/ethnic minority group participation in clinical research studies [Reference Nicholson, Schwirian and Groner16, Reference Butler20, Reference McDougall, Simpson and Friend25, Reference George, Duran and Norris26]. A recent comprehensive review of studies that included African Americans, Latinos, Asian Americans, and Pacific Islanders found that of the 44 studies reporting perceived barriers and facilitators to participating in health research between 2000 and 2011, there were many shared and distinct barriers and facilitators among the different ethnic minority groups [Reference George, Duran and Norris26]. Shared barriers to research participation included mistrust, competing demands, unintended outcomes, lack of access to information, stigma, health insurance coverage, and legal status in the USA, while shared facilitators included cultural congruence, benefits to participation, altruism, convenience of participation, and low risk in participation [Reference George, Duran and Norris26]. Distinct barriers that were unique to African Americans included a legacy of mistrust; in Asian Americans included lack of social support and acculturation; and in Pacific Islanders included misrepresentation of community. Distinct facilitators among African American studies were the design and logistics including safety assurances, trust in the researcher, having treatment options, and inclusion of diverse race/ethnic groups including Whites in the study; for Asian Americans, a distinct facilitator was endorsement from family members; and for Pacific Islanders, it was community mediation of how research findings are used and reported [Reference George, Duran and Norris26]. Our work with SAs found similar shared beliefs in the barriers and facilitators, but distinct facilitators were to have community and social network buy-in to the research. The community outreach, community recruiter, and participant referrals were the most helpful strategies in SA recruitment.
The MASALA study’s strengths are that it is the only longitudinal study of a community-based cohort of SAs in the USA, and the study’s design enables the comparison of prevalence and risk associations with four other US race/ethnic groups in the MESA study [Reference Volgman27]. However, this cohort only includes SAs from two geographic locations in the USA, and those between ages of 40 and 84 years who have no existing CVD, limiting our ability to generalize the results to all US SAs. While the demographic and socioeconomic distribution represented in this age group is grossly representative of the US SA population from Census 2010 [2], a majority of the MASALA cohort has high socioeconomic attainment and is of Indian origin.
The MASALA study had high overall study follow-up with the use of newer retention strategies that reduced participant burden associated with time and travel distance and increased spousal engagement in the study. We used different recruitment strategies to enroll a new wave of participants from sociodemographic groups that historically have had lower retention. The two recruitment methods that were most successful were participant referrals and community-based outreach by study staff. These retention and recruitment strategies may be helpful to future studies of SAs in diaspora countries. It is imperative for studies of SAs to report and analyze effective methods for engaging participants in research studies, so that there is adequate representation and generalizability of results for this diverse ethnic group.
Acknowledgments
The authors thank the study participants and other members of the study team. The MASALA study was supported by the NIH grant numbers 1R01HL093009, 2R01HL093009, and R01HL120725. Data collection at UCSF was also supported by UCSF-CTSI grant numbers UL1RR024131 and UL1TR001872, and P30DK098722. The new wave of participants was supported in part by an anonymous donor to Dr. Alka Kanaya. The sponsors did not play a significant role in the analysis, interpretation, and presentation of these results.
Disclosures
The authors have no conflict of interest to declare.







