Introduction
The spread of invasive plant species represents an urgent problem for forested ecosystems (Aronson and Handel Reference Aronson and Handel2011; Iannone et al. Reference Iannone, Heneghan, Rijal and Wise2015; Strayer et al. Reference Strayer, Eviner, Jeschke and Pace2006). Invasive species outcompete native plants via traits such as very rapid growth rates, compensatory responses to herbivory, and shade tolerance, which allows them to become dominant in the understory (Elgar et al. Reference Elgar, Freebody, Pohlman, Shoo and Catterall2014; Graebner et al. Reference Graebner, Callaway and Montesinos2012; Van Kleunen et al. Reference Van Kleunen, Weber and Fischer2010). Native tree seedling survival and growth may be impacted by nonnative plant invasions under such conditions (Malik and Bhatt Reference Malik and Bhatt2016). Although observational studies of tree seedling recruitment have demonstrated detrimental effects, the majority of studies have not employed predictive modeling (Stricker et al. Reference Stricker, Hagan and Flory2015). Precisely quantifying how plant invasions affect tree recruitment will likely be necessary to determine the ecological effects that invasions introduce to forest ecosystems.
Management Implications
Myriad factors alter composition of eastern deciduous forests in North America, especially nonnative plant species that may suppress growth and germination in native species. An invasive shrub that may be associated with lower native tree seedling densities is Japanese barberry (Berberis thunbergii DC), which spreads easily in forest understories and has been shown to alter soil chemical and microbiological traits. To date, studies have only suggested that B. thunbergii has been related to limited forest regeneration, and the effects of invasion on native plants remain understudied compared with most other invasive plants in the region. We surveyed native tree seedling densities in forest understory plots located inside and outside of B. thunbergii patches in a suburban forest of Pennsylvania, USA, and generated coefficients for models that estimate tree seedling density along gradients of B. thunbergii invasion. Dramatic reductions in native seedling densities were observed in this study, even at early stages of invasion. The models presented in this study can be used by field practitioners to predict impacts of B. thunbergii on seedling densities at larger spatial scales. Our findings suggest that effective management of this species must be performed at the earliest stages of invasion to prevent severe effects on native tree seedling recruitment and that forests invaded by B. thunbergii may face dramatic structural changes in the near future due to recruitment failure. Consequently, the significant effects we detected imply that limitations on selling, propagating, or trading B. thunbergii are highly warranted.
The diversity and productivity of eastern North American forests are changing drastically as a result of invasive species (Dobson and Blossey Reference Dobson and Blossey2015; Stinson et al. Reference Stinson, Campbell, Powell, Wolfe, Callaway, Thelen, Hallett, Prati and Klironomos2006). One of the most ubiquitous, exotic, invasive species in the region, Japanese barberry (Berberis thunbergii DC), has spread throughout much of temperate eastern North America (Ehrenfeld Reference Ehrenfeld1997, Reference Ehrenfeld1999) and has become naturalized in more than 6 Canadian provinces and 32 U.S. states (USDA, NRCS 2017). Due to its popularity as an ornamental, effective seed dispersal, and ability to reproduce through layering, B. thunbergii has established dense understory stands in forested ecosystems throughout a large geographical area (Brand et al. Reference Brand, Lehrer and Lubell2012; Manning Reference Manning1913; Silander and Klepeis Reference Silander and Klepeis1999). Berberis thunbergii can flourish in various soil and light conditions (Kourtev et al. Reference Kourtev, Ehrenfeld and Huang1998), can invade undisturbed forest understories (Harrington et al. Reference Harrington, Kujawski and Ryan2003), and is unpalatable to dominant herbivores such as white-tailed deer [Odocoileus virginianus (Zimmermann)] (Ehrenfeld Reference Ehrenfeld1997). Once established, B. thunbergii may impact ecosystem structure by altering soil pH and microbial activity, decreasing nitrogen availability, and creating a more humid and warmer microclimate during winter and cooler conditions during summer (Kourtev et al. Reference Kourtev, Ehrenfeld and Häggblom2003; Williams and Ward Reference Williams and Ward2010). Although the species remains relatively understudied compared with other invasive species (Ward et al. Reference Ward, Williams and Worthley2013), such attributes create the potential to limit forest regeneration (Harrington et al. Reference Harrington, Kujawski and Ryan2003).
Although B. thunbergii affects forest understory plants (Nuzzo et al. Reference Nuzzo, Maerz and Blossey2009; Ward et al. Reference Ward, Worthley and Williams2009, Reference Ward, Williams and Worthley2013), standard measurements for predicting native tree recruitment in invaded plots have yet to be developed. To address the need to evaluate the limitation of forest regeneration caused by B. thunbergii (Ward et al. Reference Ward, Williams and Worthley2013), we conducted an observational study on native tree seedlings within forest plots in Allegheny County, PA, that were either invaded by B. thunbergii or uninvaded. Bayesian model frameworks were used to predict the effect of invasion on native seedlings to provide robust parameters that allow forecasting of tree recruitment in invaded forests. We generated parameters to predict native seedling densities along gradients of B. thunbergii invasion intensity by treating B. thunbergii presence as a dichotomous categorical variable and along gradients of B. thunbergii invasion intensity. Models were generated using plot type (uninvaded and B. thunbergii invaded) and the degree of invasion as quantified by B. thunbergii stem count and dry mass as independent variables. Prior research suggested that native tree seedling densities would be depressed in invaded plots (Ward et al. Reference Ward, Williams and Worthley2013). Our goal, however, was to rigorously quantify such effects for use in land management and restoration applications.
Materials and Methods
Site Selection
Our study was within two temperate deciduous forest tracts located in southwestern Pennsylvania, USA: the Eden Hall Campus of Chatham University (40.6638°N, 79.9559°W) and Irwin Run Conservation Area (40.6242°N, 80.0053°W). Both forests were within close proximity to mixed-use residential zones in the suburban Pittsburgh metropolitan area and were spaced about 6 km apart. The 157-ha Eden Hall site consisted of secondary growth deciduous forest within a mosaic of agricultural land, coniferous forest, and light urban land cover, and the 29.5-ha Irwin Run site was entirely forested with deciduous species. Soils at the two sites were reported to be similar: Hazleton (Typic Dystrudepts), Clymer (Typic Hapludults), and Wharton (Aquic Hapludults) silt loams with all slopes varying between 3% and 15%. These soils have high rock content (0% and 20% fragments) and increased acidity (pH 4 to 5; Andrasko Reference Andrasko2011; Soil Survey Staff, USDA, NRCS 2017). The region experienced humid temperate domain with a mean annual precipitation of 88 cm.
The forest composition at both sites reflects the land-use history of the region. Tree assemblages in the study sites originally consisted mainly of eastern hemlock (Tsuga canadensis L.), eastern white pine (Pinus strobus L.), and American chestnut [Castanea dentata (Marsh.) Borkh.] before European colonization. However, widespread logging and land clearance for agriculture occurred across the region and at both sites. By the start of the 19th century, most land cover in the study region was agricultural, and farm abandonment began by the mid-20th century (Brown et al. Reference Brown, Johnson, Loveland and Theobald2005; Waisanen and Bliss Reference Waisanen and Bliss2002). The current, secondary growth forests are dominated by 50- to 100-year-old oak (Quercus spp.), black cherry (Prunus serotina Ehrh.), sassafras [Sassafras albidum (Nutt.) Nees.], and red maple (Acer rubrum L.). Mean canopy cover at both sites is consistently high, with a mean of 84.9±1.5% (±1 SE; C Snyder, unpublished data).
The surveys were conducted as part of two other related efforts involving the effects of B. thunbergii on ground-dwelling arthropods and mycorrhizal fungi. Although the two efforts involved differing approaches to site selection, vegetation survey methodology for both projects was consistent and concurrently conducted. Contiguous B. thunbergii understory patches with aerial cover estimated to exceed 90%, were mapped by walking the invasion perimeter with a GPS unit georeferencing contiguous invaded patches (Williams and Ward Reference Williams and Ward2010). Two methods were used to randomly select seedling survey site locations inside and outside B. thunbergii-invaded patches (Figure 1). First, we spaced 28 bordered sites at approximately 15-m increments along B. thunbergii–invaded patches, unless the perimeter abutted land cover other than mature deciduous forest, such as conifer stands. Such sites consisted of pairs, with one placed 10-m within and the other 10-m outside the B. thunbergii patches, and were chosen regardless of the overstory species composition. Second, we used GIS to create a grid of 400 by 1 m2 plots throughout the forest tracts and randomly selected 14 plots within and outside the B. thunbergii patches. At these locations (termed “interior sites”), we randomly selected three A. rubrum or Quercus spp. individuals with a diameter at breast height (DBH) between 5 and 15 cm to serve as the center point of survey sites and ensured that these sites were immediately adjacent to a source of native tree propagules. We established B. thunbergii survey sites 3 m from the base of each randomly selected tree. Consequently, our plots reflected randomly selected locations at the dynamic edge of the B. thunbergii invasion (bordered sites; n=28 control and 28 invaded sites) and well within or outside the B. thunbergii invasion (interior sites; n=42 control and 42 invaded sites).
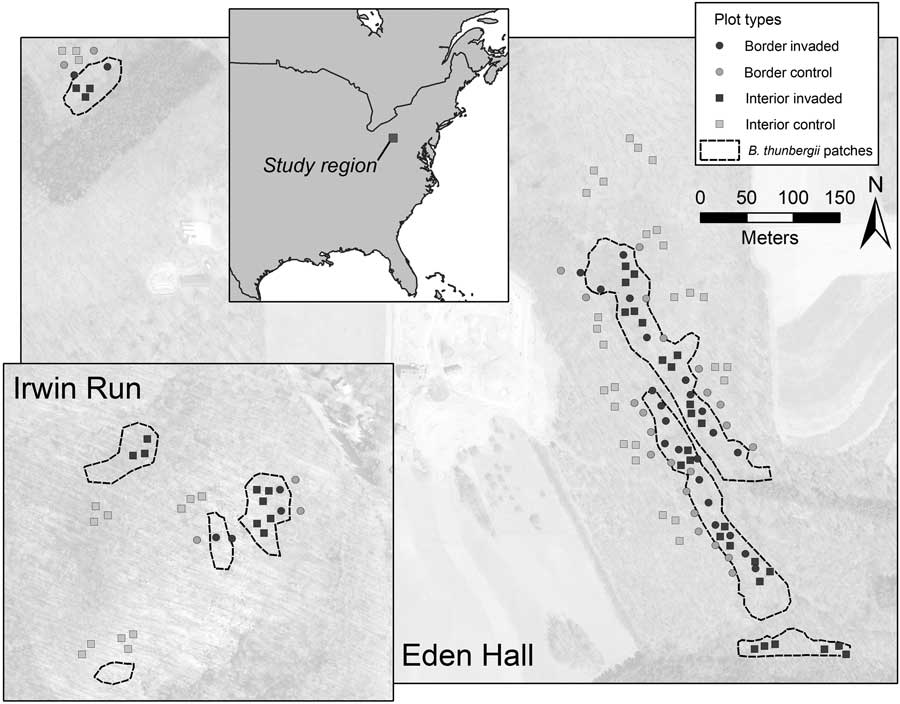
Figure 1 Map of the two study areas and plot locations. Border plots consisted of control and invaded plots 10 m within or outside of contiguous Berberis thunbergii patches. Interior plots were well within or outside B. thunbergii patches.
Field Measurements
We recorded the abundance of native tree seedlings and B. thunbergii at each plot location in July 2016. At sites along the B. thunbergii border, 4-m2 surveys were conducted within a square grid, while at sites well within or outside the border, 2-m2 surveys were conducted. Plot sizes were adjusted to account for the disparity in sample sizes for each plot type, and we later standardized these measurements by converting counts into area densities (n m−2). Within each plot, we recorded the abundance of A. rubrum, Quercus spp., and seedlings of Lauraceae (either S. albidum or spicebush [Lindera benzoin L.]) under 2.5-cm DBH. The three species groups reflected the majority (>99%) of all species of native woody plants encountered during our surveys. We also recorded the abundance of B. thunbergii at each plot by counting stems and recording stem diameter with calipers at 5 cm aboveground. The latter measurement was recorded to estimate B. thunbergii dry mass per unit area.
Statistical Analyses
To establish a B. thunbergii stem diameter to dry mass relationship, 45 stems with a minimum of 10-cm length and a range of widths were collected and returned to the lab for analysis. Diameters of these stems ranged from 1 to 25 mm. Each stem and foliage was dried at 50 C for 40 h. A simple regression model that predicted aboveground dry mass from stem width was fit to the data, with B. thunbergii dry mass predicted by Equation 1:
where DM is plant dry mass (g) and d represents the stem diameter (mm) at 5 cm aboveground as determined via least-squares regression [F(1, 42)=667.8, P<0.0001; Figure 2]. Although small, isolated patches of B. thunbergii were occasionally recorded in plots designated as control, overall stem counts and dry mass differed between plots categorized as control and invaded by one to two orders of magnitude and were significantly different as detected by a Wilcoxon rank-sum test (control: W=2.5, P<0.0001; invaded: W=3, P<0.0001).
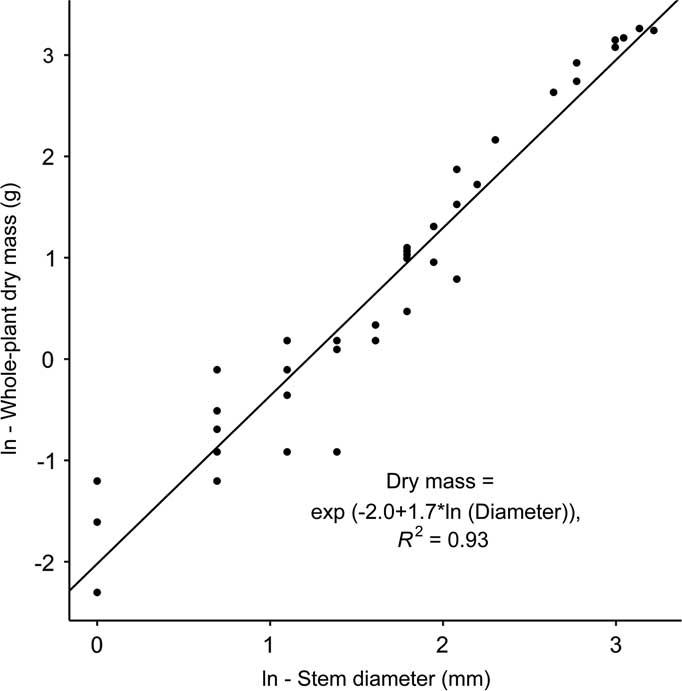
Figure 2 Berberis thunbergii aboveground dry mass as predicted by stem diameter.
Native seedling abundance between control and treatment plots and along gradients of B. thunbergii invasion intensity was predicted using Bayesian regression models. We generated models that combined all recorded seedling species (A. rubrum, Quercus spp., and Lauraceae spp.) as a dependent variable in addition to species-specific models, with the exception of Quercus spp., which were too rare in treatment plots to allow modeling. The dependent variable for all models was seedling density (n m−2), and the independent variable terms in separate models included plot type (control and invaded), B. thunbergii stem count, and B. thunbergii dry mass. After a preliminary view of the data, models of seedling densities (s) relating to B. thunbergii stem count and plant dry mass (bt) were fit to a negative-exponential equation (s=a*e (−b*bt) ), while plot type was modeled as a simple linear equation (s=a*bt + b). Prior coefficients were assumed to be normal in all models, but the posterior simulated coefficients were determined to be either negative-exponential or normal (Table 1). The data were not log transformed, because much of the data would be lost due to plots where zero seedlings were recorded (Terwei et al. Reference Terwei, Zerbe, Zeileis, Annighöfer, Kawaletz, Mölder and Ammer2013). Each model was developed using 9,000 Monte Carlo iterations, and convergence diagnostic plots produced were examined for all models to assess fit. We considered posterior values for slopes and intercepts to be significantly different if 97.5% credible intervals did not overlap with zero. Our data were applied to generate prior estimates for regression parameters, because we were unable to find precedent data that quantified B. thunbergii effects on native seedling abundance.
Table 1 Bayesian model coefficients, standard deviations (SD), and credible intervals (2.5% and 97.5%).

a Plot type models are linear, while all others represent a negative-exponential fit.
All statistical analyses were conducted in R v. 1.0.136. Bayesian analyses were produced by the program JAGS (Just Another Gibbs Sampler) v. 4.2.0 through the R package ‘rjags’ v. 4-6 (Plummer Reference Plummer2016).
Results and Discussion
Tree seedling densities were substantially lower in invaded plots compared with uninvaded plots, regardless of species (Table 1). Model outputs predicted plots with B. thunbergii to have approximately 82% lower seedling densities compared with control plots (Table 1; Figure 3). Densities decreased exponentially along gradients of increasing B. thunbergii dry mass and stem count (Table 1; Figure 4). The negative effects of invasion appeared to be expressed even in plots in the initial stages of B. thunbergii invasion. Related work has demonstrated linear declines in native plant abundance along gradients of invasive species cover (Kuebbing et al. Reference Kuebbing, Rodriguez-Cabal, Fowler, Breza, Schweitzer and Bailey2013; Levine Reference Levine2000; Stohlgren et al. Reference Stohlgren, Binkley, Chong, Kalkhan, Schell, Bull, Otsuki, Newman, Bashkin and Son1999). Considering the substantial loss of tree seedlings and significant changes to soil conditions that accompany invasion (Elgersma and Ehrenfeld Reference Elgersma and Ehrenfeld2011; Rusterholz et al. Reference Rusterholz, Küng and Baur2017; Silander and Klepeis Reference Silander and Klepeis1999), B. thunbergii appears to be acutely detrimental to native tree recruitment. The geographic extent of B. thunbergii invasion suggests that the profound decline in seedling density we detected has the potential to impact forest regeneration in eastern North America at a large spatial scale (Ehrenfeld Reference Ehrenfeld1997).
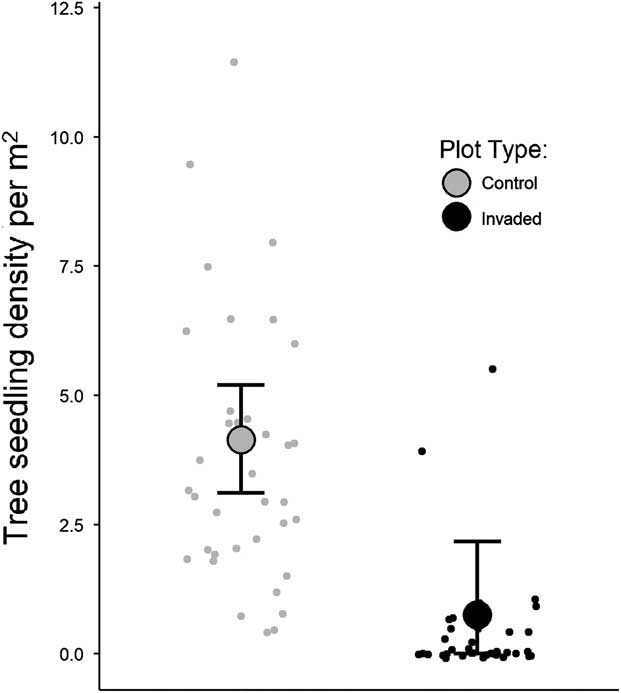
Figure 3 Comparison of observed tree seedling densities in control and invaded plots. Smaller points show seedling densities within each plot type, while the bigger points and error bars represent Bayesian estimated mean seedling densities and ±97.5% credible intervals.
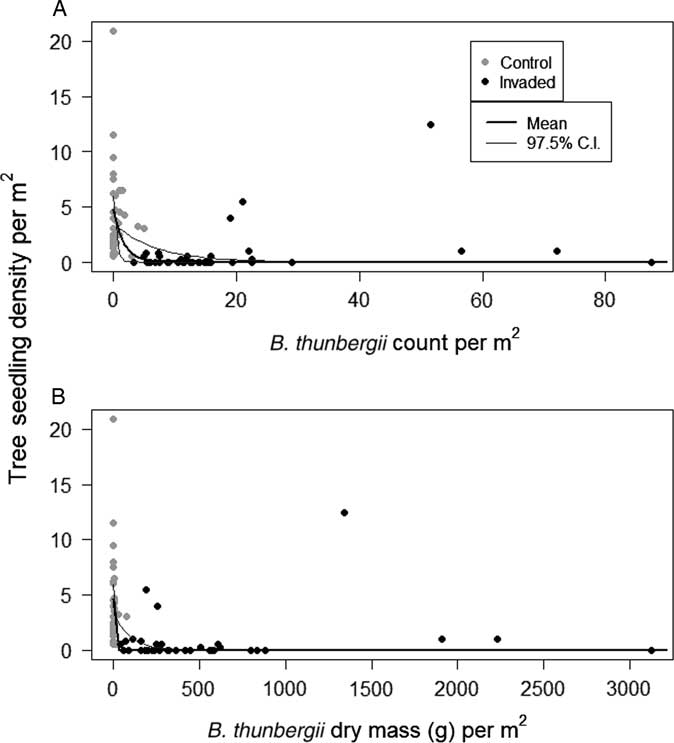
Figure 4 Observed tree seedling densities as a function of Berberis thunbergii (A) stem density and (B) predicted dry mass density within plots. Bayesian model parameters and ±97.5% credible intervals are overlaid on the data.
The severity of impact on native regeneration expressed by B. thunbergii in our observations suggests that this species represents a particularly noxious invader. Many studies investigating the impacts of invasive plant species on native flora have not detected negative effects, and if an impact was detected, the evidence was often inconclusive with respect to the mechanisms that allow the invasive to affect native biota (Gaertner et al. Reference Gaertner, Den Breeyen, Hui and Richardson2009; Gurevitch and Padilla Reference Gurevitch and Padilla2004). A global meta-analysis on how invasive plants interact with native plant communities revealed that fewer than 50% of all studied species impacted the native flora (Vilà et al. Reference Vilà, Espinar, Hejda, Hulme, Jarošík, Maron, Pergl, Schaffner, Sun and Pyšek2011). Invasion severity and impacts are often influenced by local or regional attributes, such as the history of disturbance, the ecosystem’s ability to resist the spread of exotic species, and/or the invasive characteristics of the species under consideration (Hejda et al. Reference Hejda, Pyšek and Jarošík2009; Kueffer et al. Reference Kueffer, Schumacher, Fleischmann, Edwards and Dietz2007; Pyšek et al. Reference Pyšek, Jarošík, Hulme, Pergl, Hejda, Schaffner and Vilà2012). Consequently, the severe effects we detected were likely influenced by the legacy of land-use history in our study region, particularly forests that had past agricultural use and the spread of urban land (Lundgren et al. Reference Lundgren, Small and Dreyer2004; Mosher et al. Reference Mosher, Silander and Latimer2009; Wang et al. Reference Wang, Zhang, Allen, Li, Boyer, Segerson and Silander2016). Although B. thunbergii invasion is most likely to occur in disturbed areas similar to the suburban setting where our study was conducted, invasion can be prominent in native biodiversity hot spots and relatively intact nature preserves (Ehrenfeld Reference Ehrenfeld1997; Levine Reference Levine2000; Stohlgren et al. Reference Stohlgren, Binkley, Chong, Kalkhan, Schell, Bull, Otsuki, Newman, Bashkin and Son1999). Consequently, the need to determine whether the spread of B. thunbergii communities posits a similar degree of threat to established forests is urgently needed.
Additionally, the legacy of environmental stressors in the region and related changes to trophic structure in the study forests could interact with B. thunbergii invasion to limit tree recruitment. Less than 1% of all deciduous forests in eastern North America exist in an undisturbed state and have been altered by widespread clearance for agriculture, clear-cut forestry, and land-use changes related to urbanization (Munn Reference Munn2002; Thompson et al. Reference Thompson, Carpenter, Cogbill and Foster2013). Other invasive species, such as garlic mustard [Alliaria petiolata (M. Bieb.) Cavara & Grande] (Meekins and McCarthy Reference Meekins and McCarthy1999), Amur honeysuckle [Lonicera maackii (Rupr.) Herder] (Hartman and McCarthy Reference Hartman and McCarthy2004), and Norway maple (Acer platanoides L.) (Martin et al. Reference Martin, Canham and Marks2009), impact native understory diversity and structure in the region as well, and many of these species are present in our study locations, though in lower abundance than B. thunbergii. Native seedling densities may also be reduced due to chronic grazing by O. virginianus (McGarvey et al. Reference McGarvey, Bourg, Thompson, McShea and Shen2013), which may be overpopulated in the study area due to the absence of top predators. Under normal circumstances, O. virginianus does not browse B. thunbergii due to its armed stems, and O. virginianus may avoid understories where the shrub grows in thick, recalcitrant stands. However, B. thunbergii stands may promote seed predation by providing cover for other herbivores such as worms, mice, and chipmunks (Hayes and Holzmueller Reference Hayes and Holzmueller2012; Royo and Carson Reference Royo and Carson2006).
Our results should be applied with consideration of the study limitations, yet we believe our model parameters represent a reasonable estimate of how B. thunbergii may be affecting tree recruitment in the region. The area of the study was limited to suburban forests located in a region of western Pennsylvania. Results may differ in areas with different habitats, species composition, and disturbance history, as B. thunbergii appears to spread with greater ease in forests with a legacy of land-use disturbance (DeGasperis and Motzkin Reference DeGasperis and Motzkin2007). Despite the limited spatial scale, our study sites shared similar land histories and species composition, which allowed us to control for potential confounding factors. One location-specific factor that could have affected seedling densities is herbivory. The four seedling species groups we quantified represent a small fraction of tree diversity in the region, and other species may exhibit different patterns when confronted by B. thunbergii invasion. The species that generated our models accounted for >90% of the woody species we encountered during our surveys. Additionally, the tree species that contributed the most data to our models, A. rubrum, represents one of the most resilient and abundant trees in disturbed forests in northeastern North America (Belden and Pallardy Reference Belden and Pallardy2009; Drury and Runkle Reference Drury and Runkle2006). Consequently, finding that even this species appears unable to successfully recruit in B. thunbergii–invaded habitat suggests that recruitment of any tree species will likely be severely compromised by the invasion. The tolerant nature of A. rubrum and herbivore avoidance created by B. thunbergii show that our model parameters represent conservative estimates of how the invasion affects native tree seedling densities.
Although the means by which B. thunbergii suppresses native tree recruitment remains unclear, the implications of our findings for forest management are substantial. Invasive species have been documented to suppress tree sapling recruitment by outcompeting other plants for resources such as nutrients, light, water, and space in multiple forested settings. Further research is needed to identify the specific mechanisms that allow B. thunbergii to impact native seedling recruitment with the unusual severity detected in our results. The dramatic reduction in native tree seedling densities we observed support implementing management practices to control the spread of B. thunbergii, even if the effect is less severe elsewhere. Canada and several U.S. states, including Massachusetts, have banned commercial sales of B. thunbergii as an ornamental plant in an effort to control and reduce the spread of invasion. Considering the implications for forests inherent in our study, prohibiting the sale of B. thunbergii throughout North America seems a reasonable practice to minimize the significant risks the spread of this species may represent to forests.
Acknowledgments
The authors thank the Fine Foundation, the Falk Foundation, and Chatham University for material and personnel support for this research. The Allegheny Land Trust generously provided access to their reserve at Irwin Run. Alison Molnar, Catherine Giles, and Valerie Skinner contributed valuable field assistance. No conflicts of interest have been declared.







