No CrossRef data available.
Article contents
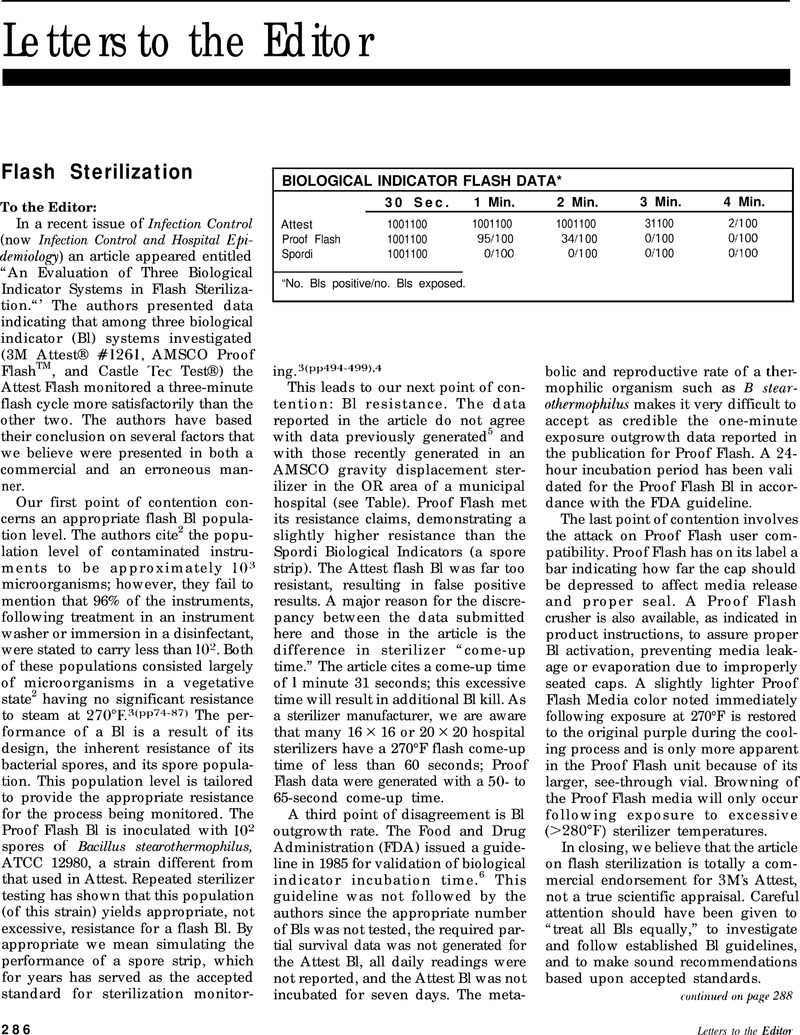
Flash Sterilization
Published online by Cambridge University Press: 07 February 2022
Abstract
An abstract is not available for this content so a preview has been provided. As you have access to this content, a full PDF is available via the ‘Save PDF’ action button.
- Type
- Departments
- Information
- Copyright
- Copyright © The Society for Healthcare Epidemiology of America 1988
References
1.
Kotilainen, HR, Gantz, NM: An evaluation of three biological indicator systems in flash sterilization. Infect Contol
1987;8:311–316.CrossRefGoogle ScholarPubMed
2.
Nyström, B: Bioburden of nondisposable surgical instruments and operating room textiles, in Gaughran, ER, Morrissiv, RF (eds): Sterilization of Medical Products. Montreal, Multiscience Publications Ltd, 1981, pp 156–163.Google Scholar
3.
Perkins, JJ: Principles and Methods of Sterilization in Health Sciences, ed 2. Springfield, IL, Charles C. Thomas, 1980.Google Scholar
4.
Plug, IJ: Syllabus for an Introductory Course in the Microbiology and Engineering of Sterilization Processes, ed 5. Minneapolis, Environmental Sterilization Laboratory, 1982, §18.7.Google Scholar
5.
Technical Report AMP-TR-19X6-009: Validation of the Modified Proof Plash Biological Indicator, 1986.Google Scholar
6.
The Center for Devices and Radiological Health: FDA Guide for Validation of Biological Indicator Incubation Time. Silver Spring, Maryland. Department of Health and Human Services, Food and Drug Administration, 1985.Google Scholar


