1. Introduction
Serpentinization is an exothermic geochemical reaction that takes place in marine and terrestrial settings where ultramafic rocks interact with seawater or meteoric waters, causing the hydration of the primary mafic mineralogy (e.g. olivine, pyroxenes) to serpentine, brucite and magnetite (e.g. Sleep et al. Reference Sleep, Meibom, Fridriksson, Coleman and Bird2004; Bach et al. Reference Bach, Paulick, Garrido, Ildefonse, Meurer and Humphris2006). This process generates large quantities of hydrogen (H2), which in turn results in the formation of abiogenic methane (CH4) through the reduction of carbon dioxide (CO2) or bicarbonate (HCO3 −) (e.g. Proskurowski et al. Reference Proskurowski, Lilley, Seewald, Früh-Green, Olson, Lupton, Sylva and Kelley2008; Etiope et al. Reference Etiope, Schoell and Hosgormez2011). Fluids deriving from the reaction are enriched in hydrogen and methane, and are usually hyperalkaline (pH = 11) (Kelley et al. Reference Kelley, Karson, Blackman, Früh-Green, Butterfield, Lilley, Olson, Schrenk, Roe, Lebon and Rivizzigno2001; Palandri & Reed, Reference Palandri and Reed2004). They can also contain high concentrations of Ca from the breakdown of Ca-rich pyroxene minerals during serpentinization (Bruni et al. Reference Bruni, Canepa, Chiodini, Cioni, Cipolli, Longinelli, Marini, Ottonello and Zuccolini2002; Chavagnac et al. Reference Chavagnac, Monnin, Ceuleneer, Boulart and Hoareau2013). When serpentinization-derived fluids come close to the seafloor or land surface, they mix with seawater or meteoric waters and carbonate minerals commonly precipitate, which are usually calcite and/or aragonite, often together with brucite (Ludwig et al. Reference Ludwig, Kelley, Butterfield, Nelson and Früh-Green2006). These authigenic carbonates can have a variety of morphologies, from veins through to highly porous chimney-like structures many metres tall (Früh-Green et al. Reference Früh-Green, Kelley, Bernasconi, Karson, Ludwig, Butterfield, Boschi and Proskurowski2003; Ludwig et al. Reference Ludwig, Kelley, Butterfield, Nelson and Früh-Green2006). Serpentinization-derived fluid emission has now been recognized in multiple geographic areas and tectonic settings where ultramafic rocks are exposed on land (as ophiolites) or in the sea. These include in the deep-sea, oceanic core complexes (e.g. Lost City on the Atlantic Massif; Kelley et al. Reference Kelley, Karson, Blackman, Früh-Green, Butterfield, Lilley, Olson, Schrenk, Roe, Lebon and Rivizzigno2001; Früh-Green et al. Reference Früh-Green, Kelley, Bernasconi, Karson, Ludwig, Butterfield, Boschi and Proskurowski2003), serpentinite mud volcanoes (e.g. Mariana fore-arc seamounts; Fryer et al. Reference Fryer, Ambos and Hussong1985) and rifted continental margins in the initial stages of ocean basin development (e.g. Iberian Margin; Agrinier et al. Reference Agrinier, Cornen, Beslier, Whitmarsh, Sawyer, Klaus and Masson1996; Klein et al. Reference Klein, Humphris, Guo, Schubotz, Schwarzenbach and Orsi2015). Examples also occur where ophiolites are in shallow-marine settings (e.g. Bay of Prony, New Caledonia and offshore Elba, Italy; Monnin et al. Reference Monnin, Chavagnac, Boulart, Ménez, Gérard, Gérard, Pisapia, Quéméneur, Erauso, Postec, Guentas-Dombrowski, Payri and Pelletier2014; Meister et al. Reference Meister, Wiedling, Lott, Bach, Kuhfuß, Wegener, Böttcher, Deusner, Lichtschlag, Bernasconi and Weber2018), and are best known from terrestrial ophiolites (e.g. Semail Ophiolite, Oman, Del Puerto Ophiolite, California and Zambales Ophiolite, Philippines; Neal & Stanger, Reference Neal and Stanger1984; Abrajano et al. Reference Abrajano, Sturchio, Bohlke, Lyon, Poreda and Stevens1988; Blank et al. Reference Blank, Green, Blake, Valley, Kita, Treiman and Dobson2009; Chavagnac et al. Reference Chavagnac, Monnin, Ceuleneer, Boulart and Hoareau2013). Whilst serpentinization-derived fluid seepage and associated carbonate mineral formation is now well known in modern settings, direct evidence for these processes in the geological record is sparse and comes largely from carbonate veins in ophiolites, often called ophicalcites (e.g. Lavoie & Chi, Reference Lavoie and Chi2010; Klein et al. Reference Klein, Humphris, Guo, Schubotz, Schwarzenbach and Orsi2015; Lafay et al. Reference Lafay, Baumgartner, Schwartz, Picazo, Montes-Hernandez and Vennemann2017; de Obeso & Kelemen, Reference de Obeso and Kelemen2018, Reference de Obeso and Kelemen2020; Cooperdock et al. Reference Cooperdock, Stockli, Kelemen and de Obeso2020). Nevertheless, the process is of great interest because of the astrobiological implications of serpentinization through the abiogenic formation of organic molecules (Proskurowski et al. Reference Proskurowski, Lilley, Seewald, Früh-Green, Olson, Lupton, Sylva and Kelley2008; Lang et al. Reference Lang, Butterfield, Schulte, Kelley and Lilley2010).
Here we describe an ancient inferred example of shallow-marine serpentinization-related seepage in the Upper Cretaceous (upper Campanian to lower Maastrichtian) Qahlah Formation of the border region between Oman and the United Arab Emirates. We suggest that the occurrence, distribution and composition (δ13C, δ18O, Mg/Ca, Sr/Ca, trace elements) of calcite cements in a small seafloor mound structure provides strong evidence for seepage of alkaline and methane-rich fluids that were released from the mantle section of the underlying Semail Ophiolite during the deposition of the Qahlah Formation. Associated hardgrounds in the Qahlah Formation may also have had a similar origin.
2. Geological setting
The Qahlah Formation crops out in the Northern Oman Mountains in the border region between Oman and the United Arab Emirates (Fig. 1a) and is the first fairly extensive sedimentary sequence to have been deposited on top of the obducted Semail Ophiolite (Smith et al. Reference Smith, Morris, Gale and Kennedy1995; Alsharan & Nasir, Reference Alsharan and Nasir1996; Abdelghany, Reference Abdelghany2006; Abbasi et al. Reference Abbasi, Hersi, Al-Harthy, Rollinson, Searle, Abbasi, Al-Lazki and Al Kindi2014). The age of the Semail Ophiolite is considered to be approximately coeval with the time of its obduction (Glennie et al. Reference Glennie, Boeuf, Clarke, Moody-Stuart, Pilaar and Reinhardt1973), and relatively little time elapsed between the final stages of this process (Searle & Cox, Reference Searle and Cox1999) and the deposition of the Qahlah Formation. The formation is time transgressive and comprises coarse clastic shallow-water sediments, variously fluviatile to marine, which are difficult to date precisely, but are usually considered to be latest Campanian or early Maastrichtian in age based on the presence of rudist bivalves, corals and especially species of large benthic foraminifera such as Loftusia (Skelton et al. Reference Skelton, Nolan, Scott, Robertson, Searle and Ries1990; Smith et al. Reference Smith, Morris, Gale and Kennedy1995; Abdelghany, Reference Abdelghany2006; Abbasi et al. Reference Abbasi, Hersi, Al-Harthy, Rollinson, Searle, Abbasi, Al-Lazki and Al Kindi2014).

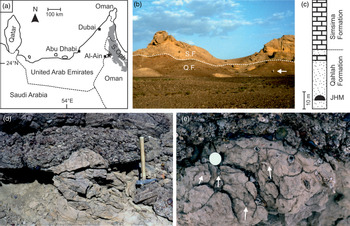
Fig. 1. (a) Location map of the Jebel Huwayyah section (black star) near Al Ain, United Arab Emirates. S. Oph. – Semail ophiolite. (b) Field image of the Qahlah (Q.F.) and the Simsima formations (S.F.) at the Jebel Huwayyah section. White arrow indicates person for scale. (c) Simplified stratigraphic section at Jebel Huwayyah showing position of the Jebel Huwayyah Mound (JHM) in lower part of the Qahlah Formation (modified from fig. 8. in Smith et al. Reference Smith, Morris, Gale and Kennedy1995). Stipple pattern – predominantly sandstones and conglomerates; brick pattern – predominantly bioclastic packstones. (d) Lateral view of JHM showing lower part of structure originating in underlying bed of medium-grained sandstone and upper part draped by overlying conglomerates. Hammer for scale = 50 cm. (e) Plan view detail of exposed top surface of the JHM showing hollow vugs and tops of vertical tubular structures (white arrows). White circle centre right of image is coin (overexposed), 25 mm in diameter.
At Jebel Huwayyah, 10 km northeast of the town of Al Ain in Abu Dhabi (Fig. 1a), up to 24 m of the Qahlah Formation crops out (Fig. 1b, c). The base of the formation and its presumed contact with the underlying Semail Ophiolite are not exposed here, but at other localities to the north, Qahlah Formation sediments lie directly on top of weathered ophiolitic rocks. The Qahlah Formation at Jebel Huwayyah consists predominantly of coarse clastic sediments (conglomerates and sandstones), sometimes cross-bedded. Unlike at most other localities, the Qahlah Formation sediments at Jebel Huwayyah have an appreciable carbonate content, including thin fringing calcite cement layers on some chert cobbles, laterally discontinuous hardgrounds formed by synsedimentary lithification in the lower and middle part of the section (Beds 2, 7 and 9 of Smith et al. Reference Smith, Morris, Gale and Kennedy1995) and marls rich in Loftusia species and other large benthic foraminifera in the upper part of the section (Smith et al. Reference Smith, Morris, Gale and Kennedy1995; Alsharan & Nasir, Reference Alsharan and Nasir1996; Wilson & Taylor, Reference Wilson and Taylor2001; Abdelghany, Reference Abdelghany2006). Abdelghany (Reference Abdelghany2006) interpreted the part of the Jebel Huwayyah section below the Loftusia beds as being deposited in fluviatile to beach environments, but this seems to contradict the finding by Smith et al. (Reference Smith, Morris, Gale and Kennedy1995) of rare shell lenses of the marine oyster Acutostrea in the basal Beds 1 and 3, and rudist fragments and pebbles encrusted with Acutostrea, bryozoans and corals in Bed 7, suggesting rather that the entire lower part of the section has a shallow-marine origin. Within Bed 2 of the Jebel Huwayyah section is a mound-shaped structure with distinctive carbonate cement-filled vugs and tubular structures (Fig. 1d, e) that is the subject of this study, which we here call the Jebel Huwayyah Mound (JHM).
3. Methods
For petrographic studies, four polished thin-sections were made of the JHM matrix sediments and tubular structures, and two Qahlah Formation hardground specimens from Bed 2. These were viewed and photographed with light microscopes at Leeds University and MARUM. Subsequently, selected polished thin-sections were stained with Feigl’s solution, Alizarin Red-S and potassium ferricyanide for identification of carbonate minerals. In addition, one unstained polished thin-section from the JHM was carbon coated and viewed with a Cameca SX-50 microprobe at Leeds University. Another thin-section was examined using a Zeiss Supra30 field emission gun scanning electron microscope (FEG-SEM) at the University of Bremen.
A number of distinctive carbonate cement phases were identified during the petrographic studies of the JHM tubular structures and, together with the matrix sediments and the hardgrounds, these were micro-drilled for C and O stable isotope analyses. CO2 for isotopic analysis was quantitatively released from carbonate samples by the standard procedure of overnight reaction in a vacuum with 100 % phosphoric acid at 100 °C. Gases were then analysed on a VG SIRA 10 mass spectrometer at the Scottish Universities Environmental Research Centre, monitoring mass:charge ratios 44, 45 and 46. Analytical raw data were corrected using standard procedures (Craig, Reference Craig1957). All isotope data are reported in the standard δ-notation in ‰ relative to Vienna Pee Dee Belemnite (V-PDB). The error of reproducibility, based on complete analysis of internal standards (including acid digestion) was ±0.1 ‰ for δ13C and ±0.2 ‰ for δ18O values. Formation temperatures were calculated after Kim & O’Neil (Reference Kim and O’Neil1997).
Minor (Mg, Si, K, Mn, Fe and Sr) and trace (Cr, Ni, Y, La, Ce, Nd, Sm, Eu, Gd, Dy, Er, Yb and Pb) elemental compositions of carbonate minerals were analysed from four polished thin-sections of the JHM with a NewWave UP193 solid state laser ablation system (λ = 193 nm) coupled to a ThermoFinnigan Element 2 sector field inductively coupled plasma mass spectrometer (ICP-MS) at the Department of Geosciences, University of Bremen. In order to avoid surface contamination, each sample was pre-ablated five times with a spot size of 120 μm. Carbonate samples were ablated by a laser beam (irradiance of ∼0.14 GW/cm2) with a pulse rate of 5 Hz and a spot size of 100 μm. Data were calibrated against the NIST SRM 612 glass standard reference (Pearce et al. Reference Pearce, Perkins, Westgate, Gorton, Jackson, Neal and Chenery1997) using 43Ca as the internal standard and assuming a Ca concentration of 40.04 wt % for calcite. Multiple laser analyses of individual calcite samples revealed a standard reproducibility of <5.5 %.
For reconstructing the past fluid Mg/Ca and Sr/Ca ratios from the JHM carbonate-filled tubes, we used the same approach as Rausch et al. (Reference Rausch, Bohm, Bach, Klügel and Eisenhauer2013). In brief, we used an average formation temperature for the various carbonate cement phases using the equation of Friedmann & O’Neil (Reference Friedmann, O’Neil and Fleischer1977), and the δ18O value of past seawater of −1 ‰ for samples older than 15 million years (Muehlenbachs, Reference Muehlenbachs1998). The distribution coefficients for Mg/Ca were calculated after Rimstidt et al. (Reference Rimstidt, Balog and Webb1998), whereas those for Sr/Ca were calculated as in Rausch et al. (Reference Rausch, Bohm, Bach, Klügel and Eisenhauer2013).
4. Results
4.a. Sedimentology and petrography
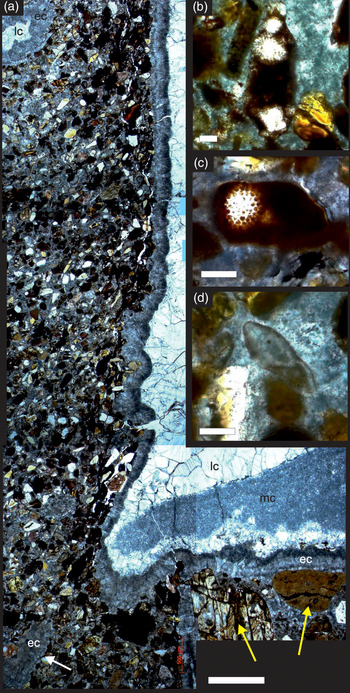
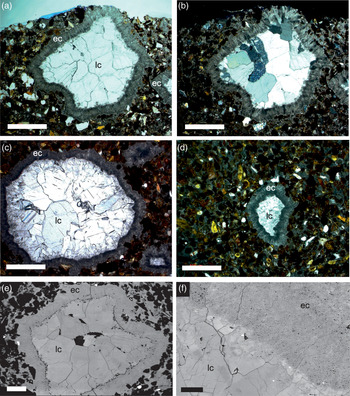
The JHM is a partially eroded, roughly spherical structure ∼1 m in diameter and is formed of medium-grained lithic sandstone cemented by inclusion-rich calcite (Fig. 1d, e). The mound originates from an underlying bed of sandstone, forming a positive structure draped by overlying conglomerates (Fig. 1d). The sand grains forming the JHM are sub-angular to sub-rounded in shape and are a mixture of lithologies (Figs 2, 3), including in order of decreasing abundance: haematite-stained serpentine, quartz, opaque chert (sometimes with radiolarian fossils; Fig. 2b, c), magnetite, chromium spinel, biotite and micritized biogenic carbonate grains (Fig. 2d). Numerous hollow vugs and tubular structures occur within the JHM, but are not found in the underlying or host sandstone, or the overlying conglomerates (Fig. 1e). The tubes are roughly cylindrical, 0.5 to 7 mm in diameter (Fig. 3), and have an orientation that is roughly perpendicular to the base of the mound (Fig. 1e). The tubes have a visible length of up to 80 mm, but because of the eroded nature of the JHM some could have originally been longer. One tube cut longitudinally has a flat base containing a small number of significantly larger fresh serpentine grains (up to 2 mm in length), and below this base, a number of converging, thin calcite-filled channels (Fig. 2a). This tube also shows a distinctive orientation of the surrounding sand grains, which over the space of a few millimetres become increasingly rotated towards the tube, so that some grains come to lie parallel with the tube wall (Fig. 2a). The vugs and tubes in the JHM are lined by two main phases of calcite cement: (1) an early inclusion-rich, fibrous calcite that forms isopachous rims 0.2–0.3 mm in thickness lining tube walls, which we hereafter refer to as early cement, and (2) a later phase of inclusion-free equant, blocky calcite, which we hereafter refer to as late cement (Figs 2a, 3). The early cement we interpret to be the same calcite generation that cements the grains forming the mound (Fig. 2b–d) on the basis of appearance, continuity from grain-cementing to isopachous rims (Fig. 2a) and stable isotope values (Table 1). In some tubes the late cement reveals patches of microsparitic calcite (Fig. 2a), and in some of the larger diameter tubes and vugs the late cement does not entirely fill their interiors, leaving hollow spaces (Figs 1e, 3a).

Fig. 2. Photomicrographs of petrographic thin-sections from the Jebel Huwayyah Mound (all in plane polarized light). (a) Longitudinal section through one of the fluid conduits, top uppermost in the image. At the base of the conduit are two large, fresh serpentine grains (yellow arrows) and a calcite-filled channel (white arrow). Abbreviations: ec – early cement; mc – microsparitic cement; lc – late cement. (b, c) Two opaque chert grains containing radiolarian fossils. (d) Micritized formaminiferan test. Scale bars: (a) = 500 µm; (b–d) = 100 µm.

Fig. 3. Transverse sections of Jebel Huwayyah Mound fluid conduits. (a, b) Section of one conduit with particularly angular outline in (a) plane polarized light and (b) cross-polarized light. (c) Conduit with more circular outline. (d) Smaller conduit with very irregular outline. (e, f) Back-scattered electron micrographs of conduit showing late-stage cement calcite crystals increasing in size to centre of conduit and hollow interior (black space); and, (f) in detail, clear differentiation between inclusion-rich early cement and inclusion-free late cement. Abbreviations: ec – early cement; lc – late cement. Scale bars: (a–e) = 500 µm; (f) = 100 µm.
Table 1. δ18O and δ13C values from the Jebel Huwayyah Mound (JHM) fluid conduit cements, matrix cements and Qahlah Formation (QF) hardgrounds

Formation temperatures were computed from δ18OSMOW values using the calibration by Kim & O’Neill (Reference Kim and O’Neil1997).
4.b. Carbon (δ13C) and oxygen (δ18O) stable isotopes
The δ13C values of the early cement in the JHM tubes range from −4.2 ‰ to −3.3 ‰ (n = 4), whereas the δ13C values of the late cement range from –7.9 ‰ to −5.3 ‰ (n = 9; Table 1; Fig. 4). A single δ13C value from the microsparitic cement (−5.8 ‰) is similar to the range of the late cement with which it is associated. The δ13C values of the calcite cementing the grains forming the mound are between −3.5 ‰ and −3.3 ‰ (n = 3). The δ18O values fall between −3.1 ‰ and −1.3 ‰ for the early calcite cement, between −3.1 ‰ and −1.1 ‰ for the late cement, and between −3.0 ‰ and −2.2 ‰ for calcite cementing the mound; one sample of microsparitic cement yielded a δ18O value of −1.5 ‰. The δ13C values of the Qahlah Formation hardgrounds range from −9.1 ‰ to −4.8 ‰, and corresponding δ18O values are between −4.1 ‰ and −0.3 ‰. The oxygen isotopic compositions were used to estimate the precipitation temperature of calcite (Table 1), using the empirical relation between δ18Ocalcite – δ18Owater and temperature by Kim & O’Neil (Reference Kim and O’Neil1997).

Fig. 4. Cross plot (δ13C v. δ18O) of Jebel Huwayyah Mound fluid conduit cements, mound matrix and Qahlah Formation hardgrounds. Data from Table 1.
4.c. Mg/Ca and Sr/Ca ratios
The elemental composition of the calcite-filled tubular structures in the JHM reveal distinct Mg/Ca and Sr/Ca ratios for the two main cement phases (Fig. 5; online Supplementary Material Table S1). The early cement has Mg/Ca ratios of between 9.53 and 19.44 mmol/mol and Sr/Ca ratios of 0.25 to 0.47 mmol/mol, whilst the Mg/Ca ratios for the late cement range from 0.85 to 6.45 mmol/mol and the Sr/Ca ratios are between 0.04 and 0.17 mmol/mol. The Mg/Ca and Sr/Ca ratios for the microsparitic cement are, with one exception, intermediate between the values of the early and late cements. The reconstructed Mg/Ca and Sr/Ca ratios of the parental fluid from which the calcites precipitated are listed in online Supplementary Material Table S1.

Fig. 5. Sr/Ca v. Mg/Ca ratios of the Jebel Huwayyah Mound fluid conduit cements. Data from online Supplementary Material Table S1.
4.d. Rare earth elements and yttrium
Rare earth element (REE) and yttrium (Y) concentrations of the calcite cements (Fig. 6; online Supplementary Material Table S2) show an overall flat post-Archaean Australian shale (PAAS)-normalized pattern for the late phase with small positive Y anomalies. The early cement shows slight light REE depleted patterns and lacks a positive Y anomaly. Both of the main cement phases show weak negative Ce anomalies.

Fig. 6. Rare earth element and Y patterns of the Jebel Huwayyah Mound fluid conduit cements (early phase and late phase only). Data were normalized to post-Archaean Australian shale (PAAS; Taylor & McLennan, Reference Taylor and McLennan1985). Note that the data presented are averages of eight to ten laser-ablation spots (see online Supplementary Material Table S2).
5. Interpretations
5.a. Jebel Huwayyah Mound formation
The formation of the JHM began with the deposition in a shallow-marine setting of the medium-grained lithic sandstone that forms the matrix of the mound. Soon after this, on a small area of seafloor, calcite cement precipitated that bound the grains together, forming a positive mound-shaped structure. Because of the relatively low concentration of Sr in this cement phase (Fig. 5; online Supplementary Material Table S2), we infer that the mineralogy of this early cement phase was originally calcite and not aragonite; calcite resulting from the recrystallization of aragonite tends to retain high Sr contents in the order of several thousand ppm (Buggisch & Krumm, Reference Buggisch and Krumm2005; Peckmann et al. Reference Peckmann, Campbell, Walliser and Reitner2007). We interpret the tubes within the mound to represent conduits through which the fluids flowed, from which the early-stage calcite cement precipitated. Proof of upward fluid flow comes from the rotated sand grains close to the edges of the fluid conduits and winnowed finer grains from the sediment, leaving only coarse grains in the flat bases of some of the conduits (Fig. 2a). The rims of inclusion-rich fibrous calcite (the early cement) in the vugs and tubes precipitated at the same time as the mound-forming matrix cements.
An alternative explanation for the grain rotation in the tubes might be the movement of animals through the lithic sediment to produce burrows prior to cementation. However, burrows tend to have much more regular shapes (particularly in transverse sections) than the JHM conduits, and are almost invariably sediment filled (e.g. Bromley, Reference Bromley1996). The JHM tubes and vugs are also not post-cementation animal borings, because the cements and grains at the periphery of the conduit walls are not truncated, as can be seen in borings in hardgrounds and reworked cobbles higher up in the Jebel Huwayyah section (Wilson & Taylor, Reference Wilson and Taylor2001). Plant root traces can be shaped like the JHM tubes, but again, most are filled by later sediments, and may contain carbonaceous remnants (Gregory et al. Reference Gregory, Martin and Campbell2004), which the JHM conduits lack completely. In addition, animal burrows and plant root traces cannot explain the presence of vugs with the same cement linings as the tubes in the JHM, or the grain winnowing within the conduits. For these reasons we hereafter refer to the JHM tubes as fluid conduits.
Sometime after the formation of the JHM, the second phase of inclusion-free blocky calcite (the late cement) and associated microsparitic cement precipitated in the vugs and fluid conduits from later stage fluids circulating within the mound. The larger size of the late cement crystals compared to the early cement crystals suggests that the precipitation of the former occurred less rapidly than the latter. The timing of the late cement formation is difficult to ascertain and most probably occurred after the JHM was covered by a layer of conglomerate and later sediments.
5.b. Origin and nature of the JHM cement-forming fluids
Here we use the stable isotope and element composition of the calcite cements in the JHM to reconstruct the origin and nature of the fluids from which they formed, starting with the early cement. The Mg/Ca and Sr/Ca ratios of the reconstructed parent fluid from which this cement precipitated are close or slightly lower than 1000 mmol/mol, and the corresponding Sr/Ca ratios cluster around 4 mmol/mol, respectively (online Supplementary Material Table S1). These ratios match well with the range of reconstructed Late Cretaceous seawater compositions (Mg/Ca = ∼1000 mmol/mol, Sr/Ca = 2 to 6 mmol/mol) that are based on calcite veins from ocean crust in the flanks of mid-ocean ridges (Coggon et al. Reference Coggon, Teagle, Smith-Duque, Alt and Cooper2010; Rausch et al. Reference Rausch, Bohm, Bach, Klügel and Eisenhauer2013), as well as with theoretical models for the Late Cretaceous (Hardie, Reference Hardie1996; Wallmann, Reference Wallmann2001). In contrast, the δ13C values (−4.2 ‰ to −3.3 ‰) from the early cement are around 5 ‰ lower than would be expected for calcite precipitated in equilibrium with dissolved inorganic carbon (DIC) of Cretaceous seawater (+2 ‰; Wilson & Opdyke, Reference Wilson and Opdyke1996; Prokoph et al. Reference Prokoph, Shields and Veizer2008), and indeed the values recorded from the Maastrichtian Simsima Formation at Qalhat, NE Oman (0 to +2 ‰; Schlüter et al. Reference Schlüter, Steuber, Parente and Mutterlose2008). An explanation for the negative δ13C values in the JHM early cement requires the mixing of the contemporary seawater DIC pool (modern seawater δ13CDIC = ∼0 ‰; Zeebe & Wolf-Gladrow, Reference Zeebe and Wolf-Gladrow2001) with an isotopically lighter source of dissolved DIC. One common source of DIC with low δ13C values reflecting 13C depletion is the oxidation of organic carbon (Irwin et al. Reference Irwin, Curtis and Coleman1977). However, there is no obvious source of significant organic material (e.g. carbon-rich shales) in the sedimentary sequence hosting the JHM, as the Qahlah Formation rests directly on the crystalline rocks of the Semail Ophiolite, so another source of isotopically light DIC needs to be invoked. We suggest that the ophiolite itself could have been this source, through the production of abiogenic methane by serpentinization of peridotite. Methane in modern serpentinite-derived fluids in both the deep-sea and in ophiolite environments has negative δ13C values (−11.9 ‰ at Lost City, −10.3 ‰ at Logatchev, −16.7 ‰ at Rainbow, −18 ‰ at Elba and −7.7 ‰ in the Zambales ophiolite; Abrajano et al. Reference Abrajano, Sturchio, Kennedy, Lyon, Muehlenbachs and Bohlke1990; Lilley et al. Reference Lilley, Butterfield, Olson, Lupton, Macko and McDuff1993; Charlou et al. Reference Charlou, Donval, Fouquet, Jean-Baptiste and Holm2002; Proskurowski et al. Reference Proskurowski, Lilley, Seewald, Früh-Green, Olson, Lupton, Sylva and Kelley2008; Meister et al. Reference Meister, Wiedling, Lott, Bach, Kuhfuß, Wegener, Böttcher, Deusner, Lichtschlag, Bernasconi and Weber2018; Sciarra et al. Reference Sciarra, Saroni, Etiope, Coltorti, Mazzarini, Lott, Grassa and Italiano2019). Some of this methane will be oxidized close to the seafloor to produce 13C-depleted DIC, and the high pH and Ca concentrations in the serpentinite-derived fluids promote carbonate precipitation on mixing with seawater (Palandri & Reed, Reference Palandri and Reed2004; Proskurowski et al. Reference Proskurowski, Lilley, Seewald, Früh-Green, Olson, Lupton, Sylva and Kelley2008). These carbonates usually (but not always) have negative δ13C values, often with the same range as the JHM cements. The abundance of serpentine sand grains in the JHM matrix is ample evidence for serpentinization of the Semail Ophiolite to have occurred in the Jebel Huwayyah area during Late Cretaceous time, certainly prior to the formation of the sand grains, and, as we here suggest, during the deposition of the overlying Qahlah Formation.
The late cement phase precipitated from a fluid with lower Mg/Ca and Sr/Ca ratios than the early cement phase (Fig. 5). Serpentinization fluids show low Mg and high Ca concentrations relative to seawater (e.g. Kelley et al. Reference Kelley, Karson, Blackman, Früh-Green, Butterfield, Lilley, Olson, Schrenk, Roe, Lebon and Rivizzigno2001). Moreover, the lower Mg/Ca and Sr/Ca ratios of the JHM late cements are similar to those of serpentinite-hosted calcite from the Logatchev hydrothermal system, which Eickmann et al. (Reference Eickmann, Bach, Rosner and Peckmann2009) interpreted to reflect mixing between seawater and a hydrothermal fluid. Applying this evidence to our model for the JHM, we infer that the fluids from which the late cement phase precipitated comprised a greater proportion of serpentinization-derived fluids to seawater than the early cement phase. This is corroborated by the more negative δ13C values of the late cement (Fig. 4), which indicates a greater influence of methane oxidation on the DIC pool from which this mineral phase precipitated.
Because there is a trend from higher to lower Mg/Ca and Sr/Ca ratios in carbonate cements during diagenesis (e.g. Tucker & Wright Reference Tucker and Wright1990; Joseph et al. Reference Joseph, Campbell, Torres, Martin, Pohlman, Riedel and Rose2013), it is possible that the JHM late cement had a diagenetic origin, from burial diagenesis and/or interaction with meteoric water during early cementation. However, this is rather unlikely for several reasons. First because the early cement and the late cement of the JHM tubes have similar δ18O values (−3.1 ‰ and −1.3 ‰ versus −3.1 ‰ and −1.1 ‰), possibly indicating that the oxygen stable isotope composition of the fluids and formation temperatures did not change much from the time when the early cement formed to the time when the late cement mostly occluded the fluid conduits. Second, we think that later diagenetic alteration would affect both the early and late JMH cement phases, leading to homogenization of δ13C values, and Mg/Ca and Sr/Ca ratios between the early and later calcite phases. We also note that Schlüter et al. (Reference Schlüter, Steuber, Parente and Mutterlose2008) recorded δ18O values from the carbonate-rich Simsima Formation as low as −8 ‰, which they interpreted as showing diagenetic alteration; these values are considerably more negative than any found in the JHM cements.
The Mg/Ca and Sr/Ca ratios for the microsparitic cement phase are largely intermediate between the early and late cements (Fig. 5). This may indicate that the microsparitic cement precipitated from a fluid with a composition between that from which the early and late cements precipitated. However, the single δ13C value for the microsparitic cement sits in the field of the late-stage cement (Fig. 4), so it is likely the microsparitic cement precipitating fluids were more related to this later phase of cementation in the JHM.
The calculated formation temperatures for the all the JHM cement phases (Table 1) based on their δ18O values range between 14 and 24 °C (mean 19.9; n = 17), assuming a seawater value of −1 ‰ for an ice-free Late Cretaceous (Veizer et al. Reference Veizer, Bruckschen, Pawelick, Diener, Podlaha, Carden, Jasper, Korte, Strauss, Azmy and Ala1997; Prokoph et al. Reference Prokoph, Shields and Veizer2008). This range is at the lower end or below temperature estimates for Maastrichtian tropical sea-surface temperatures of 20 to 32 °C from planktonic foraminifera (Pearson et al. Reference Pearson, Ditchfield, Singano, Harcourt-Brown, Nicholas, Olsson, Shackelton and Hall2001; Zeebe, Reference Zeebe2001), 27 to 32 °C from rudist bivalve aragonite and magnesian calcite cements (Wilson & Opdyke, Reference Wilson and Opdyke1996), and a minimum of 25 °C using the TEX86 proxy (Alsenz et al. Reference Alsenz, Regnery, Ashckenazi-Polivoda, Meilijson, Ron-Yankovich, Abramovich, Illner, Almogi-Labin, Feinstein, Berner and Püttmann2013). However, these calculated JHM palaeotemperatures should be taken with caution, as we do not know the δ18O value(s) for the inferred serpentinization-derived fluids, nor the degree of mixing with contemporary seawater in the JHM during cement precipitation. Further, the cement δ18O values could have been overprinted by later diagenetic events (e.g. Tong et al. Reference Tong, Wang, Peckmann, Cao, Chen, Zhou and Chen2016), although see the arguments above about a possible diagenetic interpretation.
Support from PAAS-normalized REEs for our interpretation of serpentinization-derived fluids being involved in the formation of the JHM is equivocal. The REE and Y patterns of the early- and late-stage calcite cements (Fig. 6) show subtle differences between the two generations. The early cement phase displays very uniform patterns with a slight heavy REE (HREE) enrichment. The lack of a positive Y anomaly suggests that the fluid from which the early cement precipitated was modified by water–rock reactions. The late cement does show a positive Y anomaly and has lower HREE concentrations. It is not straightforward to reconcile the differences in the REE patterns with the variability in Mg/Ca ratios and δ13C values. This is in part due to the fact that REE and Y systematics of fluids involved in low-temperature serpentinization have not been investigated to date.
To sum up, we have a number of geochemical lines of evidence that the cements forming the JHM were precipitated from serpentinization-derived fluids mixed to a greater or lesser extent with Maastrichtian seawater for a period of time during the deposition of the Qahlah Formation. The formation of the JHM tubes is therefore analogous to fluid-induced chimney formation on the modern ocean floor, where the interplay between ascending venting fluids and seawater forms chimneys recording a progressive change from a seawater-like signature in the outermost part to a lesser seawater component in the inner part (Eickmann et al. Reference Eickmann, Thorseth, Peters, Strauss, Bröcker and Pedersen2014). Following from this, and with reference to the chemistry of modern serpentinization-related seeps, we infer that the JMH serpentinization-derived fluids were alkaline and rich in molecular hydrogen, abiogenic methane and Ca.
5.c. Qahlah Formation hardgrounds
The δ13C values of the hardground cements are similar to those of the JHM late cement, although one of the values (−9.1 ‰) is the most negative of all the carbonates we measured from the Qahlah Formation (Fig. 4), and also more negative than any marine hardgrounds in the geological record documented by Erhardt et al. (Reference Erhardt, Turchyn, Dickson, Sadekov, Taylor, Wilson, Scott and Schrag2020), with the lowest value of −5.15 ‰ from the Lower Cretaceous of Oman. From this we infer, again, that the fluid from which the hardground cements precipitated was not pure seawater. Although we have no supporting elemental data, we suggest that this isotopically lighter DIC source was from the same serpentinization-derived fluid that contributed to the formation of the JHM cements. Some support for this idea is that the hardgrounds are not laterally continuous (i.e. do not represent periods of basin-scale emergence or non-deposition) and are only found in the Jebel Huwayyah section, not in other outcrops of the Qahlah Formation (see Section 2). If this interpretation is correct, then it suggests serpentinization-derived fluid flow was active for a considerable time during the formation of the Qahlah Formation, but this was localized palaeogeographically to the Jebel Huwayyah area. Such localization would be expected given that sites of modern serpentinite-related seepage are geographically constrained to small areas of land-based ophiolites and submarine mantle rock exposures (see Section 1).
6. Discussion and conclusions
There are several implications of our interpretation that serpentinization-derived fluids were involved in the formation of the JHM and conduit-filling cements, and perhaps also the Jebel Huwayyah hardgrounds. First, it expands the temporal duration of serpentinization-related seepage in the Semail Ophiolite, and the morphological diversity of resulting carbonate mineral structures. Second, it is, to the best of our knowledge, the only ancient example of a carbonate mineralized seafloor feature formed from serpentinization-related seepage from shallow-marine settings.
Today, the Semail Ophiolite is host to numerous terrestrial hyperalkaline springs, the famous Omani ‘Blue Pools’ (e.g. Neal & Stanger, Reference Neal and Stanger1984; Chavagnac et al. Reference Chavagnac, Monnin, Ceuleneer, Boulart and Hoareau2013; Giampouras et al. Reference Giampouras, Garrido, Bach, Los, Fussmann, Monien and García-Ruiz2020), where serpentinization-derived fluids mix with meteoric waters. Earlier evidence of serpentinization of the Semail Ophiolite comes from Wadi Fins in SE Oman where there are veins of calcite and subordinate dolomite within a host rock of serpentinized peridotite, associated with deep clastic dykes filled with sedimentary carbonates (de Obeso & Kelemen, Reference de Obeso and Kelemen2018, Reference de Obeso and Kelemen2020; Cooperdock et al. Reference Cooperdock, Stockli, Kelemen and de Obeso2020). These calcite veins have 87Sr/86Sr ratios indicative of an age around the Cretaceous–Paleocene boundary (de Obeso & Kelemen, Reference de Obeso and Kelemen2018) and (U–Th)/He values in hydrothermal magnetite in the veins, which give an age estimate of 15 ± 4 Ma, equating to the Miocene (Cooperdock et al. Reference Cooperdock, Stockli, Kelemen and de Obeso2020). Cooperdock et al. (Reference Cooperdock, Stockli, Kelemen and de Obeso2020) interpreted these calcite veins as having formed over a considerable period of time through the interaction of the Semail Ophiolite mantle peridotites with pore fluids derived from overlying Cretaceous and Paleocene limestone formations. The JHM shows that similar processes started earlier, possibly in late Campanian to early Maastrichtian time, soon after the end of ophiolite obduction onto the Arabian continental margin (Searle & Cox, Reference Searle and Cox1999). Post-emplacement serpentinization of the Semail Ophiolite likely occurred even before this, as lateritic debris in conglomerates at the base of Maastrichtian marine sediments show that parts of the ophiolite were raised above sea-level prior to the early Maastrichtian transgression and subjected to sub-aerial weathering (Coleman, Reference Coleman1981; Al-Khirbash, Reference Al-Khirbash2015). It seems likely that some serpentinization could have occurred during this weathering phase. Further, based on δ18O data Gregory & Taylor (Reference Gregory and Taylor1981) suggested that some serpentinization of the Semail Ophiolite mantle sequence was taking place even as it was being formed. The reconstructed composition of the JHM fluids differs from the modern Semail Ophiolite hyperalkaline springs in being a mixture of serpentinization-derived fluids with seawater rather than with meteoric waters and the precipitation of calcite only, rather than calcite, aragonite and brucite. Because brucite is retro-soluble, it is conceivable that this mineral was precipitated during the formation of the JHM and was then replaced during diagenesis. However, there is no petrological evidence (e.g. pseudomorphic minerals) in the studied JHM thin-sections for this sort of replacement process. Further, brucite has not been recorded in the Wadi Fins calcite veins, which de Obeso & Kelemen (Reference de Obeso and Kelemen2018) interpreted as an indication of non-isochemical serpentinization at that site. The JHM calcite cement δ13C values are more negative than those from the Wadi Fins calcite veins (−1.3 to +0.6 ‰), which correspond rather to the values from the overlying Simsima Formation (de Obeso & Kelemen, Reference de Obeso and Kelemen2018).
Close modern analogues to the environment in which the JHM formed are the shallow-marine serpentinization-derived fluid and gas seeps found in the Bay of Prony, New Caledonia and off the island of Elba, Italy. The Prony hydrothermal field (PHF) comprises a suite of fluid seeps in a marine lagoon, in the intertidal zone and onshore springs (Launay & Fontes, Reference Launay and Fontes1985; Monnin et al. Reference Monnin, Chavagnac, Boulart, Ménez, Gérard, Gérard, Pisapia, Quéméneur, Erauso, Postec, Guentas-Dombrowski, Payri and Pelletier2014; Pisapia et al. Reference Pisapia, Gérard, Gérard, Lecourt, Lang, Pelletier, Payri, Monnin, Guentas, Postec, Quéméneur, Erauso and Ménez2017). The marine seeps are precipitating submarine carbonate structures with complex morphologies in the 30 m and 50 m depth range, including the well-known Aiguille de Prony, which is 35 m tall and reaches to within 2 m of the water surface. These structures are highly porous and are formed largely of calcite, with increasing amounts of brucite mixed with Mg-carbonates and aragonite towards their interiors (Pisapia et al. Reference Pisapia, Gérard, Gérard, Lecourt, Lang, Pelletier, Payri, Monnin, Guentas, Postec, Quéméneur, Erauso and Ménez2017). The PHF fluids have a high pH and high concentration of aqueous calcium and hydroxide, with only traces of other solutes (Monnin et al. Reference Monnin, Chavagnac, Boulart, Ménez, Gérard, Gérard, Pisapia, Quéméneur, Erauso, Postec, Guentas-Dombrowski, Payri and Pelletier2014). The low salinity values agree with fluids derived from the mixing of meteoric water with fluids derived from serpentinization, even though in most places in the Bay of Prony fluids are now discharging into the sea and ultimately mix with seawater. In this respect they differ from the composition of the fluids which formed the JHM, which we infer to have had a large seawater component, at least for the early cement phase.
Another analogous modern setting to the JHM are sites offshore Elba in the Tyrrhenian Sea (Meister et al. Reference Meister, Wiedling, Lott, Bach, Kuhfuß, Wegener, Böttcher, Deusner, Lichtschlag, Bernasconi and Weber2018; Sciarra et al. Reference Sciarra, Saroni, Etiope, Coltorti, Mazzarini, Lott, Grassa and Italiano2019). At the Pomonte site, gas emission occurs across an area of 1000 m2 of seafloor in 10–13 m water depth through quartz-rich, organic-poor sands, deposited on top of rocks of the Ligurian Ophiolite, which locally include serpentinized peridotite. Gas bubbles are enriched in methane (δ13Cmethane ∼ −18 ‰) and hydrogen, and have a very low CO2 content. The gas seeps are associated with areas of discoloured sediment containing semi-lithified carbonate crusts, at between 20 and 40 cm depth within the sediment. The crusts are formed of spherulitic, fibrous aragonite that cements sand grains and sometimes bryozoans and seagrass rhizome fibres. The crusts have δ13C values of between −17 and +2 ‰ and δ18O values of approximately +1.5 ‰. Based on the decrease in pore water sulphate concentrations at the gas seeps relative to seawater, increases in sulphide and DIC, and the negative δ13C values, Meister et al. (Reference Meister, Wiedling, Lott, Bach, Kuhfuß, Wegener, Böttcher, Deusner, Lichtschlag, Bernasconi and Weber2018) inferred that the Elba seep carbonate cements are the product of microbial sulphate-dependent anaerobic oxidation of abiotic methane (AOM).
This scenario of AOM-related carbonate precipitation in shallow-marine sediments can be applied to the hardgrounds in the Qahlah Formation, although the δ13C values of the latter are more positive than some of the Elba seep carbonate values. The JHM itself contrasts with the Elba example because the carbonates are calcite rather than aragonite, which may reflect the predominantly calcite precipitation in Cretaceous seas (Sandberg, Reference Sandberg1983), rather than any fundamental differences in seepage fluid composition. Other differences are that the JHM shows evidence of several stages of fluid mixing and cement precipitation to form seafloor rather than subsurface features as in Elba. Nonetheless, there are enough similarities between the New Caledonian and Elba examples and the JHM to indicate the latter (and possibly the Qahlah Formation hardgrounds) is another example of shallow-marine serpentinite-derived seepage, and therefore adds a data point in the geological record to our knowledge of this interesting phenomenon.
Acknowledgements
We thank Sebastian Flotow (Bremen) for the preparation of petrographic thin-sections and Heike Anders (Bremen) for help and assistance with LA-ICP-MS analyses. Mark Wilson (Wooster) is thanked by P.D.T. for his help during fieldwork. This work was supported by the Deutsche Forschungsgemeinschaft (B.E., grant number 5441317) and a Fellowship from the Hanse-Wissenschaftskolleg (HWK) to C.T.S.L. to support visits to the University of Bremen. Comments by Bas van de Schootbrugge and an anonymous reviewer are gratefully acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0016756821000121