Article contents
Oral microbiota in cancer: could the bad guy turn good with application of polyphenols?
Published online by Cambridge University Press: 13 December 2022
Abstract
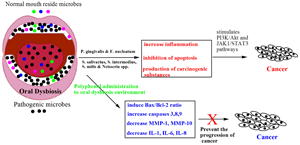
The human oral cavity is comprised of dynamic and polynomial microbes which uniquely reside in the microenvironments of oral cavities. The cumulative functions of the symbiotic microbial communities maintain normal homeostasis; however, a shifted microbiota yields a dysbiosis state, which produces local and systemic diseases including dental caries, periodontitis, cancer, obesity and diabetes. Recent research reports claim that an association occurs between oral dysbiosis and the progression of different types of cancers including oral, gastric and pancreatic ones. Different mechanisms are proposed for the development of cancer, such as induction of inflammatory reactions, production of carcinogenic materials and alteration of the immune system. Medications are available to treat these associated diseases; however, the current strategies may further worsen the disease by unwanted side effects. Natural-derived polyphenol molecules significantly inhibit a wide range of systemic diseases with fewer side effects. In this review, we have displayed the functions of the oral microbes and we have extended the report regarding the role of polyphenols in oral microbiota to maintain healthy conditions and prevention of diseases with emphasis on the treatment of oral microbiota-associated cancer.
- Type
- Review
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
References
- 2
- Cited by



