Introduction
The aetiology of autism spectrum disorder (ASD), a group of neurodevelopmental disorders that cause persistent social impairment with difficulties in communication and repetitive behavioural patterns, involves both genetic and environmental factors (Ref. Reference Hodges, Fealko and Soares1). Prevalence of ASD rapidly increased from 1 out of 150 U.S. children in 2000, to 1 out of 68 children in 2012 and 1 out of 59 children in 2018 (Ref. Reference Baio2). A recent systematic review and meta-analysis found that the male-to-female ratio of autism in children is approximately 3 to 1, which could be lower due to diagnostic gender bias that is less likely to diagnose females meeting ASD criteria compared to males (Ref. Reference Loomes, Hull and Mandy3). By 2016, black children in the United States were 1.5 times more likely to be identified with ASD than white or Hispanic children, suggesting that ASD is associated with social determinants of health involving socio-economic status, housing, physical environment and racial discrimination (Ref. Reference Shaw4).
Figure 1 shows clinical signs and symptoms of ASD, based on information provided by the American Academy of Pediatrics Council on Children With Disabilities (Ref. Reference Johnson and Myers5). Age of onset of ASD symptoms ranges from the first year of infancy up to 2–3 years (Ref. Reference Hyman, Levy and Myers6). Adolescents with ASD have a higher risk of developing depression, anxiety and attention-deficit hyperactivity disorder (Ref. Reference Accardo, Pontes and Pontes7). With no known cause, treatments for ASD patients are mostly symptomatic to improve quality of life and daily functioning (Ref. Reference Politte8).

Figure 1. Signs and symptoms of autism spectrum disorder, based on American Academy of Pediatrics Council on Children With Disabilities (Ref. Reference Johnson and Myers5).
Among potential environmental factors in ASD aetiology, dietary associations with ASD merit further investigations. In particular, studies of neurodevelopmental disorders from exposure to toxic levels of dietary phosphate are lacking. Dietary phosphorus in the form of inorganic phosphate (Pi) is an essential mineral that is regulated in the body by a sensitive network of hormones released by the kidneys, intestines, parathyroid glands and bone (Ref. Reference Brown and Razzaque9). Dysregulation of phosphate metabolism can lead to the accumulation of excess phosphate in the body causing a condition known as phosphate toxicity. Exposure to phosphate toxicity can negatively impact almost every major organ system in the body, including the central nervous system, with possible implications for the aetiology of neurodevelopmental disorders in ASD.
Method
The present narrative review used a grounded theory literature-review method (Ref. Reference Wolfswinkel, Furtmueller and Wilderom10) to search, retrieve and analyse research findings using keywords related to dysregulated phosphate metabolism, phosphate toxicity and ASD. Grounded theory in this method replaces subjectivity and conjecture with a rigorous method to synthesise new information grounded in evidence. Through an iterative process of comparative analysis, findings from the research literature were developed into themes and formed into associated relationships, and additional keywords were searched to follow the trail of evidence and fill in knowledge gaps. The strength of this method's bottom-up inductive approach lies in discovering potential breakthrough knowledge grounded in findings from published literature (Ref. Reference Brown11).
Nevertheless, ‘it is impossible to not be influenced by the background knowledge that one has’ (Ref. Reference Wolfswinkel, Furtmueller and Wilderom10), and limitations of this method, as in most other research methods, include potential selection and information biases that influence data selection, analysis, interpretation and presentation of the research findings (Ref. Reference Tripepi12). Findings may not be generalisable (external validity), and the researcher must successfully defend the work's internal validity under critical appraisal by peer experts. In the present paper, synthesised associations and proposals provide novel insights and directions for future research linking ASD aetiology and dysregulated phosphate metabolism.
ASD, cancer and phosphate
A retrospective cohort study of over 8000 children and adolescents with autistic disorder estimated that cancer incidence within the cohort was 94% higher than the expected cancer incidence (standardised incidence ratio 1.94) (Ref. Reference Chiang13). A more recent population-based study of 2.3 million individuals from Nordic countries found that overall risk from any cancer increased in ASD, but only in individuals with comorbid intellectual disability and/or birth defects (Ref. Reference Liu14), implying involvement of other causative factors such as chromosomal abnormalities related to mutations in PTEN (Phosphatase and Tensin Homolog), a tumour suppressor phosphatase (Ref. Reference Liu14). Phosphatases and protein kinases are enzymes that modulate cellular proteins by catalysing the transfer of phosphate in opposing directions (Ref. Reference Cheng15). A protein kinase catalyses the transfer of phosphate from ATP or GTP to phosphorylate a protein while a phosphatase catalyses the transfer of phosphate from a phosphoprotein to a water molecule. Similar to cell signalling in cancer (Ref. Reference Brown and Razzaque16), autism has been linked to an altered balance between phosphoinositide kinases and the counteracting effect of phosphatases in neuronal membranes (Ref. Reference Gross17).
Phosphorus is a growth-rate limiting factor in tumorigenesis (Ref. Reference Kuang, Nagy and Elser18). High dietary phosphate activates phosphoinositide 3-kinase (PI3 K) which phosphorylates Akt (protein kinase B) leading to activation of mTOR kinase that upregulates protein synthesis in cancer cell proliferation while suppressing apoptosis (Ref. Reference Guertin and Sabatini19). High dietary phosphate also suppresses the counteracting effect of PTEN on PI3 K (Ref. Reference Jin20). A similar cell-proliferation effect is seen in autism and epilepsy as PI3 K activates tuberous sclerosis complex 1/2 (TSC1/2) which activates mTOR kinase (Refs Reference Davis21, Reference Saxena and Sampson22). PTEN mutations in autism and epilepsy also suppress counteraction of PI3 K, leading to tumours and neurodevelopmental disorders in PTEN hamartoma tumour syndrome (Ref. Reference Yehia, Keel and Eng23). A study of brain analyses found that white matter in PTEN-ASD patients had a specific overgrowth pattern, with poor white matter development, reduced processing speed, increased deficits in working memory and extensive intellectual limitations (Ref. Reference Frazier24). Results of these brain tissue analyses imply that high concentrations of Pi may accumulate in the white matter of PTEN-ASD patients, who make up approximately 20% of children with ASD (Ref. Reference Busch25), and future studies should investigate Pi brain concentrations in PTEN-ASD patients.
Of relevance, phosphorus 31 magnetic resonance spectroscopy (MRS) was used in an earlier study to detect high concentrations of Pi in temporal lobe epilepsy (Ref. Reference Laxer26). More recently, increased Pi was detected in gliomas using MRS (Ref. Reference Peter and Nandhan27), and dysregulated expression of the gene PHD-finger protein 3 (PHF3) in glioblastoma overlaps with similar dysregulated gene expression of PHF3 in autism (Ref. Reference Appel28). Increased benign glioma risk of the central nervous system in children is also associated with neurofibromatosis type 1 (NF1) (Ref. Reference Costa and Gutmann29), and autistic behaviour is elevated in children with NF1 (Ref. Reference Chisholm30).
Feasibly, as in cancer, future research may show that high dietary phosphate associated with phosphate toxicity activates PI3 K and causes PTEN mutations in autism and epilepsy, leading to overgrowth and defective neuronal connectivity. Of relevance, phosphoric acid used as a common food additive was found to cause genotoxic damage to DNA in human lymphocytes (Ref. Reference Yilmaz31), which is an epigenetic factor that could increase PTEN mutations. Furthermore, lower urinary excretion of phosphoric acid was found in children with autism compared to controls (Ref. Reference Chen32), possibly indicating higher retention of phosphoric acid in body tissue with greater genotoxic damage in children with autism. More research is needed in the role of dysregulated phosphate metabolism in PTEN mutations in ASD.
ASD, dietary phosphate and gliosis
Infants who have been breastfed for long periods have better cognitive development and lower risk of autistic traits (Ref. Reference Boucher33). Phosphorus is about six-times lower in human breast milk (15 mg per 100 ml) than in cow milk (Ref. Reference Platt and Moncrieff34), and phosphorus plasma levels were lower and calcium levels higher in breastfed newborn babies compared to newborn babies fed unmodified cow milk (Ref. Reference Oppé and Redstone35). In infancy, high phosphorus, sodium, potassium, protein and chloride intake from inappropriate consumption of whole cow milk places a burden on developing kidneys' solute load (Ref. Reference Leung and Sauve36). These findings suggest that breastfeeding is associated with lower risk of renal burden and reduced incidence of dysregulated phosphate metabolism potentially related to autistic traits.
Casein in cow milk, a phosphoprotein with phosphate groups bonded to amino acid side groups, makes up about 80% of cow milk protein (Ref. Reference Sarode, Caballero, Finglas and Toldrá37). Accordingly, dietary patterns that reduce casein also reduce phosphorus, which may account for many of the suggested benefits of casein-free diets in children with ASD (Ref. Reference Quan38). Although gluten protein in grain does not contain phosphorus, bread and baked goods are often high in phosphorus and phosphate additives (Ref. Reference León, Sullivan and Sehgal39). While a negative effect from gluten ingestion remains unproven in ASD, gluten-free diets are suggested to have beneficial effects in ASD (Ref. Reference Croall, Hoggard and Hadjivassiliou40), which might be attributed to reductions in overall intake of phosphorus in bread and baked goods.
The rise in ASD has been suggested to originate in changes to the gut microbiome from increasing consumption of additives in processed food. Abdelli et al. found that exposing human neuron stem cells to the common food preservative propionic acid increased inflammatory responses and gliosis (excessive proliferation of fibrous support cells in the central nervous system) as occurs in neuro-circuitry dysfunction in ASD (Ref. Reference Abdelli, Samsam and Naser41). Interestingly, the researchers also found that propionic acid decreased levels of PTEN by about 50% compared to controls, which allowed levels of activated p-Akt (phosphorylated Akt) to increase and stimulate glial cell proliferation. Abdelli et al. also noted decreased neurite outgrowth in neuronal cells, which differentiate into dendrites or axons, and decreased axonal expansion was also noted which the researchers attributed to a physical barrier caused by excessive glia. The researchers proposed that dysregulated outgrowth and expansion explained disrupted communication between neuronal cells reported in ASD.
Figure 2 is a schematic diagram proposing that Pi from dysregulated phosphate metabolism phosphorylates Akt, leading to gliosis and neuro-circuitry disturbance in ASD. Future research should test this mechanism with in vitro experiments that compare glial cell proliferation in cultures exposed to concentrations of Pi, as well as in vivo experiments comparing levels of dietary phosphate and gliosis in lab animals. Although rodents are commonly used to model genetic mutations in autism, limited use of non-human primates comes closer to matching autistic behaviour in humans (Ref. Reference Katsnelson42).

Figure 2. Neuro-circuitry disturbance in autism spectrum disorder. Based on https://upload.wikimedia.org/wikipedia/commons/7/73/Blausen_0672_NeuralTissue.png.
ASD, dysregulated phosphate and immune/neuroinflammatory responses
Abdelli et al. measured immune responses and neuroinflammation in human neural stem cells from exposure to propionic acid, including increased levels of inflammatory cytokines such as tumour necrosis factor alpha, with smaller increases in the anti-inflammatory cytokine interleukin 10 (Ref. Reference Abdelli, Samsam and Naser41). Rodriguez and Kern proposed that reducing neuroinflammation and microglial activation could lead to improvements in neurodevelopment outcomes in people with ASD (Ref. Reference Rodriguez and Kern43).
Feasibly, immune responses related to neuro-circuitry disturbances in ASD may be caused by dysregulated phosphate. The following text compares abnormalities of natural killer cells (NK-cells), T-cells, B-cells, macrophages and monocytes in patients with ASD (Ref. Reference Gevezova44) with similar immune responses in conditions involving dysregulated phosphate. Although NK-cell proliferation was reported to increase in ASD, the NK-cells had reduced functioning ability (Ref. Reference Ashwood45). Normally, NK-cells regulate neuron and glia functions, including proliferation and neurite outgrowth (Ref. Reference Ebrahimi Meimand, Rostam-Abadi and Rezaei46), implying that NK-cell dysfunction and neuro-circuitry disturbances in ASD may share a common cause from exposure to phosphate toxicity. Of relevance, NK-cells are dysfunctional in end-stage kidney disease patients receiving dialysis therapy (Ref. Reference Nagai47), which is a procedure that helps control dysregulated phosphate (Ref. Reference Cupisti48).
T-cell abnormalities in ASD include reduced proliferation of T-helper cells (Th) and reduced regulatory T cells (Treg) with upregulation of Th2 cells (Ref. Reference Gevezova44). Interestingly, Th2 cells are involved in hypersensitivity to food antigens (Ref. Reference Ellenbogen49) which may include hypersensitivity from exposure to excessive dietary phosphate. Of relevance to increased skin sensitivity in ASD (Ref. Reference Jameson50), high serum phosphate is associated with skin itching—pruritus (Ref. Reference Swarna51), and pruritus is associated with increased Th2 cytokines (Ref. Reference Garcovich52). T cell exhaustion also occurs in kidney failure (Ref. Reference Hartzell53), which is associated with harmful levels of serum phosphate (Ref. Reference Rastogi54).
Children with ASD also have higher levels of pro-inflammatory B-cells [34]. B-cell infiltrates that occur in renal interstitial inflammation (Ref. Reference Heller55) are potentially related to B-cell infiltrates in kidney burden from phosphate toxicity (Ref. Reference Mironov, Atfi and Razzaque56). Macrophage and monocyte immune responses are also common in ASD (Ref. Reference Gevezova44) and in conditions with dysregulated phosphate such as kidney disease. For example, accumulation of macrophages and monocytes in renal tissue causes inflammation and is associated with renal tissue damage, scarring and reduced kidney function (Ref. Reference Wang57).
Dysregulated Pi in chronic kidney disease (CKD) also shares many other enzymes, immune responses and inflammatory markers with ASD. Compared to children with normal development, children with ASD were found to have elevated protein expression for IL-1β, IL-4, IL-9, interferon gamma (IFN-γ), Janus kinase 1 (JAK1), phosphorylated JAK1 (pJAK1), signal transducer and activator of transcription 5 (STAT5) and pSTAT5 (Ref. Reference Ahmad58). Similarly, patients with diabetic nephropathy, a leading cause of end-stage renal disease with dysregulated Pi, have increased expression of IL-1β and IL-4 (Ref. Reference Araújo59), and overproduction of IL-9 and IFN-γ was found in patients with acute glomerular injury of the kidneys (Ref. Reference Kim60). Additionally, JAK1 expression is enhanced in diabetic nephropathy (Ref. Reference Brosius and He61), phosphorylated JAK1 stimulates production of large amounts of FGF23, a regulator of phosphate metabolism (Ref. Reference Daryadel62), abnormal amounts of STAT5 are expressed in autosomal dominant polycystic kidney disease (Ref. Reference Fragiadaki63), and pSTAT5 is increased in focal segmental glomerulosclerosis of the kidneys (Ref. Reference Tao64).
Compared to children with normal development, children with ASD showed elevated expression of chemokine receptors in CD4 + T cells, including chemokine receptor 2 + (CXCR2 + ), CXCR3 + , CXCR5 + and CXCR7 + and C-C motif chemokine receptor 3 + (CCR3 + ), CCR5 + , CCR7 + and CCR9+ (Ref. Reference Ahmad65). Correspondingly, chemokine receptors CXCR1-7 are highly expressed in clear cell renal cell carcinoma (Ref. Reference Wu66), CCR3 is upregulated in renal cell carcinoma (Ref. Reference Jöhrer67), CCR5 + contributes to the pathogenesis of CKD (Ref. Reference Fuhro68), and children with CKD have increased expression of CCR7 and other chemokine receptors (Ref. Reference Szczepańska69). Furthermore, CCR9 + is associated with food allergens (Ref. Reference Castan70), which may include reactions to exposure to excessive dietary phosphate.
Nuclear factor-erythroid 2 related factor 2 (Nrf2), a transcription factor that protects immune cells against inflammation and oxidation, was reduced in children with autism (Ref. Reference Nadeem71). Similarly, impaired production of Nrf2 in CKD increases oxidation and inflammation leading to nephritis (Ref. Reference Ruiz72). Severity of symptoms in children with autism is associated with upregulation of IL-6 receptors and IL-17A in CD4 + T cells (Ref. Reference Nadeem73), and upregulated IL-17A expression in neutrophils increases inflammation and oxidation in children with autism (Ref. Reference Nadeem74). Likewise, IL-17A plays a central role in kidney disease pathogenesis (Ref. Reference Cortvrindt75) while IL-6 increases expression of FGF23 in acute and CKD (Ref. Reference Durlacher-Betzer76). Autism in children is also associated with elevated expression of IL-16 in CD4 + , CD8 + , CD14 + , CCR3 + and CXCR7 + cells (Ref. Reference Ahmad77). Similarly, IL-16 contributes to inflammation of the kidneys which can lead to impaired kidney function (Ref. Reference Tavener, Jewell and Panickar78). Furthermore, immune dysfunction in children with autism is associated with upregulated T cell immunoglobulin and mucin domain 3 (TIM-3) (Ref. Reference Ahmad79), and overexpression of TIM-3 is associated with severe kidney inflammation (Ref. Reference Lu80).
Research is needed to explore common mechanisms that explain how dysregulated phosphate metabolism may contribute to immune and inflammatory responses in both kidney disease and ASD. Other environmental factors and toxic conditions can also contribute to ASD—nevertheless, evidence implicating dysregulated phosphate metabolism in ASD warrants further investigation.
ASD, phosphate additives and ultraprocessed food
U.S. consumption of ultra-processed foods, defined as industrially made, ready-to-eat or heat-and-serve products that contain food additives and generally lack whole foods, has continuously increased over the past two decades (Ref. Reference Juul81), concurrent with increases in ASD prevalence. Children with ASD often prefer highly processed foods with phosphate additives, including chicken nuggets, pizza, macaroni with cheese, breakfast cereals, pancakes, waffles, hot dogs, crackers and bread, with less preference for fresh produce (Ref. Reference Mayes and Zickgraf82). Compared to children with typical development, children with ASD were found to consume approximately 20–30% more ultra-processed food (Ref. Reference Buro, Kakkad and Gray83). Of relevance, consumption of ultraprocessed food high in inorganic phosphates is associated with renal function decline in older adults (Ref. Reference Rey-García84).
Additionally, a recent systematic review and meta-analysis found a 58% increased risk of obesity associated with ASD in children compared to controls (Ref. Reference Sammels85). Higher prevalence of childhood obesity in ASD may be related to higher energy intake and weight gain caused by ultra-processed food intake, as demonstrated in a controlled study of ultra-processed food consumed by adults with obesity (Ref. Reference Hall86). Maternal intake of ultra-processed food is also associated with increased obesity in offspring (Ref. Reference Wang87). Furthermore, a national cross-sectional study found that ultra-processed food consumption is greater among younger aged, lower income and less educated Americans (Ref. Reference Baraldi88), and more research is needed to compare these demographic findings with ASD prevalence. Because neurodevelopment in newborns is critically influenced by maternal prenatal diet, which is suggested to play a role in ASD aetiology (Ref. Reference Zhong89), research should investigate whether younger mothers with less education, lower income, obesity and greater consumption of ultra-processed food have a higher risk of ASD in their offspring.
Many sugar-sweetened beverages (SSBs), such as colas, contain phosphoric acid, and increased consumption of SSBs is associated with stronger and more difficult emotional problems in ASD (Ref. Reference Tan90). In addition, baking powder, chocolate, cocoa and beer are common sources of dietary phosphate that may contribute to excessive phosphate intake (Ref. Reference Picard91). A 2019 national survey of food items in Finland (representative of Europe) found phosphate additives in 36% of sampled foods, and 17 different phosphate additives were observed overall (Ref. Reference Tuominen, Karp and Itkonen92). Phosphorus-containing food additives are listed in Table 1.
Table 1. Phosphorus-containing food additives
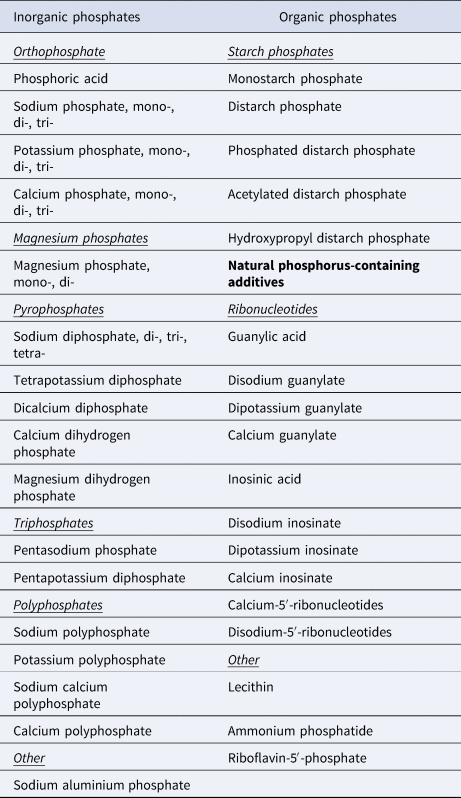
Based on Tuominen et al. (Ref. Reference Tuominen, Karp and Itkonen92).
Sodium aluminium phosphate is commonly added to baking powder and processed cheese (Ref. Reference Saiyed and Yokel93). Excessive environmental exposure to aluminium within the body causes aluminium ions to bind with phosphate, which interferes with phosphorylation and dephosphorylation mechanisms in kinase and phosphatase enzymes, and contributes to mitochondrial dysfunction, microglia activation and immune and neuroinflammatory responses (Ref. Reference Morris, Puri and Frye94). Compared to controls, greater levels of aluminium in brain tissue have been detected in autism and neurodegenerative diseases (Ref. Reference Exley and Clarkson95), and intracellular aluminium in autism is concentrated within microglial cells and other non-neuron cells of brain tissue (Ref. Reference Mold96).
In addition to higher dietary phosphate intake, average concentrations of urinary phosphorus excretion are 34% lower in children with ASD (Ref. Reference Qureshi97), suggesting positive net phosphate absorption and tissue storage in ASD. Importantly, serum phosphate levels do not always accurately reflect tissue Pi storage levels (Ref. Reference Osuka and Razzaque98), and abnormal amounts of phosphate shifted from serum to tissue storage could explain hypophosphatemia found in some children with ASD compared to healthy children (Ref. Reference Afroz99). More studies are needed using phosphorus 31 MRS for detection of phosphate tissue storage in autism during brain development (Ref. Reference Minshew, Pettegrew and Garreau100).
Additionally, dietary fat is free of phosphorus, and lower phosphorus intake levels may explain benefits in ASD from high-fat ketogenic diets (Ref. Reference Li101). Ketogenic diets are effective in reducing or preventing seizures in epilepsy (Ref. Reference D'Andrea Meira102), and ASD is associated with epilepsy (Ref. Reference Besag103), implying that both ASD and epilepsy may share a common pathophysiological cause related to dysregulated phosphate metabolism and phosphate toxicity.
Evidence suggests that excessive dietary protein intake above optimal levels for neurodevelopment can be as harmful as deficient protein intake in perinatal care (Ref. Reference Barreault104). Of relevance, each gram of protein from dietary sources is directly related to approximately 12–14 mg phosphorus (Ref. Reference D'Alessandro, Piccoli and Cupisti105). Consequently, dietary protein intake levels above optimal amounts for neurodevelopment are likely to involve excessive amounts of phosphorus with increased risk of dysregulated phosphate metabolism.
Higher ASD prevalence in males is consistent with higher mean dietary phosphorus intake in U.S. male children aged 2–11 years compared to females, with significantly higher phosphorus intake in male adolescents aged 12–19 years (Ref. Reference Moshfegh, Kovalchik and Clemens106). Interestingly, some children with ASD show improvements in behaviour during fever (Ref. Reference Grzadzinski107). Coincidently, tumours also regress following fever (Ref. Reference Køstner108), and fever is associated with reduced appetite (Ref. 109), inferring lower food intake. Accordingly, reduction in dietary phosphate intake associated with fevers may act as a mediating factor that temporarily improves behaviours in ASD and may reduce tumours as lower phosphate levels limit the rate of cancer cell growth.
ASD, diabetes and dysregulated phosphate
Large studies have found that ASD is associated with increased prevalence of type 1 and type 2 diabetes mellitus (Ref. Reference Tromans110). Of relevance, neuronal degeneration and other complications in diabetes are associated with dysregulated phosphate metabolism (Ref. Reference Brown111). These findings infer that dysregulated phosphate metabolism is a pathophysiological mechanism common to diabetes mellitus and neuronal degeneration in ASD. Maternal diabetes in rats induces autism-like behaviour in offspring (Ref. Reference Wang112). In humans, maternal obesity comorbid with diabetes is associated with a greater risk of ASD in offspring compared to obesity or diabetes alone (Ref. Reference Li113). This finding implies that high dietary phosphate intake in obesity may interact with dysregulated phosphate metabolism in diabetes, leading to greater associated risk of ASD. Further research is needed to confirm an interactive relationship in ASD between obesity and diabetes mediated by excessive phosphate intake.
Prevalence of neurodevelopmental disorders, including ASD, is higher in children with type 1 diabetes, and diabetic nephropathy and retinopathy are also higher in type 1 diabetic children with neurodevelopment disorders (Ref. Reference Liu114). Of relevance, children with ASD have a higher risk of ophthalmologic diagnosis (Ref. Reference Chang115) and 25% of adults with ASD were found to have CKD (Ref. Reference Miot116), providing further support for dysregulated phosphate metabolism as a common pathophysiological cause in diabetic complications and ASD. Furthermore, low muscle tone or hypotonia is an established marker of individuals with ASD (Ref. Reference Gabis117). Similarly, hypotonia affects children with dysregulated phosphate metabolism in CKD (Ref. Reference Abd El Naby118), and hypotonia is associated with increased mortality in patients with renal failure receiving dialysis therapy for hyperphosphatemia (Ref. Reference Wanic-Kossowska and Czekalski119).
ASD, mitochondrial dysfunction and tauopathy
Mitochondrial dysfunction is associated with pathogenesis of ASD, and occurs in up to 80% of children with ASD (Ref. Reference Castora120). Coincidentally, mitochondrial dysfunction is associated with dysregulated phosphate metabolism in the pathogenesis of diabetes (Ref. Reference Brown111) and Parkinson's disease (Ref. Reference Brown121). Precipitates of calcium phosphate collect within the mitochondria matrix of pancreatic beta cells and substantia nigra cells that produce insulin and dopamine in diabetes and Parkinson's disease, respectively, which interfere with the function of complex I in the electron transfer chain during oxidative phosphorylation. The result of mitochondrial dysfunction in cells includes reduced ATP biosynthesis, apoptosis and cell death. Importantly, the majority of children with autism in an exploratory controlled study had below-normal values of complex 1 activity (Ref. Reference Giulivi122). Research is needed to examine the pathophysiologic effect of dysregulated phosphate metabolism, phosphate toxicity and calcium phosphate precipitation in mitochondrial dysfunction affecting neuronal cells of individuals with ASD.
In tauopathies associated with neurodegenerative diseases like Alzheimer disease and Parkinson's disease (Ref. Reference Zhang123), hyperphosphorylation of tau, a protein that assists in neuron cell maturation and cytoarchitecture, causes microtubule dysfunction which is linked with tau aggregation and neurofibrillary tangles (Ref. Reference Xia, Prokop and Giasson124). Autism-like behaviours have been linked to increased levels of frontotemporal lobe tau and neurofibrillary pathology in late-life dementia (Ref. Reference Rhodus125). Genetically lowering or removing tau in mouse models prevented or reduced key features of ASD, including abnormally enlarged head size, epilepsy and overactivation of the PI3 K/Akt/mTOR signalling pathway, which resulted in disinhibition of PTEN (Ref. Reference Tai126). Drugs that interfere with tau phosphorylation are proposed to treat tauopathies (Ref. Reference Xia, Prokop and Giasson124), but increased attention should focus on dietary interventions that reduce excessive phosphate intake, which may act as a rate-limiting factor to reduce tau hyperphosphorylation in the aetiology of neurodegenerative diseases and neurodevelopmental diseases like ASD. Importantly, hyperphosphorylation of tau is associated with glial-tau pathology in glial cells (Ref. Reference Kahlson and Colodner127), further implicating hyperphosphorylation as a cause of excessive glial cell proliferation and gliosis, which disrupts neuro-circuitry in ASD.
ASD, vitamin D, bone disorders and cardiovascular disease
The bioactive form of vitamin D, 1,25(OH)2D3, also known as calcitriol, is synthesised by the kidneys from the storage form of vitamin D, 25-hydroxy vitamin D, 25(OH)D3 (Ref. Reference Brown and Razzaque9). Calcitriol increases intestinal absorption of Pi during digestion, raising serum phosphorus levels as needed. To reduce excessive levels of serum Pi, the kidneys produce less bioactive vitamin D, which decreases phosphorus absorption in the intestines. Low levels of 25(OH)D3, are common in ASD (Ref. Reference Petruzzelli128), and lower serum levels of calcitriol and calcium were also found in ASD compared to normal controls (Ref. Reference Meguid129)—a potential biomarker indicating a kidney response in ASD that attempts to reduce serum Pi by lowering phosphate intestinal absorption when dietary phosphate intake is excessive.
Other endocrine hormones downregulate serum Pi by increasing renal phosphaturia, including fibroblast growth factor 23 (FGF23) released from bone and parathyroid hormone (PTH) released from the parathyroid glands (Ref. Reference Brown and Razzaque9). A recent case study reported elevated serum PTH and serum phosphate in a 14-year old boy with ASD having hypocalemic seizures following a prolonged diet of processed snack foods (Ref. Reference Kota, Kumar and Bargman130). Researchers also found structural changes in the frontal lobes associated with increased FGF23, likely in response to high levels of Pi which could also explain increased vascular calcification and microangiopathic lesions found in the brain leading to white matter loss (Ref. Reference Marebwa131). In general the brain's frontal lobes control social and cognitive behaviour, and frontal cortex damage is known to lead to the onset of persistent and repetitive behaviour, insistence for sameness, and impulsive behaviours which are clinical features of ASD (Ref. Reference Khadem-Reza and Zare132). Interestingly, low serum levels of basic fibroblast growth factor, fibroblast growth factor 2 (FGF2), which regulates neurodevelopment, were found in children with ASD (Ref. Reference Esnafoglu and Ayyıldız133). Future research is needed to investigate how dysregulated Pi impacts high FGF23 and low FGF2 levels in young children with ASD.
Odds of hip fractures in a case-control study were higher in children and adults with ASD compared to non-ASD individuals, and odds of fractures of the forearm and spine were greater in women with ASD (Ref. Reference Neumeyer134). Compared to typically developing children, a meta-analysis found that children with ASD had up to 13% lower bone mineral density of the total body, which is associated with increased risk of fractures (Ref. Reference Rostami Haji Abadi135). Osteoporosis and fracture are also associated with dysregulated phosphate metabolism in CKD-mineral and bone disease (CKD-MBD) (Ref. Reference Pazianas and Miller136), suggesting a common cause with fractures in ASD.
Bone mineral disorders related to dysregulated phosphate metabolism also impact poor oral health and periodontal disease, and dental plaque associated with gingivitis and periodontitis often forms from excessive calcium phosphate in saliva (Ref. Reference Brown137). A meta-analysis in children and young adults with ASD found a pooled prevalence of approximately 70% for periodontal disease (Ref. Reference da Silva138), implicating phosphate toxicity in ASD as a potential pathophysiological factor in poor oral health.
Autism is also associated with endothelial dysfunction of the arterial system, which contributes to disruption of neurovascular coupling mechanisms in neurodevelopment (Ref. Reference Ouellette139). In another potential link between ASD and dysregulated phosphate metabolism, hyperphosphatemia has a deleterious effect on endothelial dysfunction (Ref. Reference Stevens140). Of relevance, risk factors for cardiovascular disease (CVD) are increased in CKD, and the primary treatment goal in CKD is control of phosphate load (Ref. Reference Ritter and Slatopolsky141). Increased risk for heart failure and atrial fibrillation in CKD (Ref. Reference Rehm142) overlaps with similar risk for heart failure and atrial fibrillation in ASD (Ref. Reference Sun143). Moreover, a higher risk of death in people with coronary disease was associated with higher serum phosphate levels (Ref. Reference Tonelli144). Even people with normal renal function were found to have an increased risk of cardiovascular events associated with higher serum phosphate (Ref. Reference McGovern145). Although many environmental factors that contribute to ASD pathogenesis are shared with kidney disease, studies are lacking that investigate the association of paediatric kidney disease with ASD in children (Ref. Reference Clothier and Absoud146), especially related to dysregulated phosphate metabolism.
Directed acyclic graph
Figure 3 is a directed acyclic graph (DAG), often used in epidemiological and clinical research to illustrate causal pathways and mediating factors that help explain the association of outcomes with exposures to risk factors (Ref. Reference Digitale, Martin and Glymour147). The DAG in Figure 3 shows that dysregulated phosphate metabolism is a potential mediating factor causatively linking (solid arrows) associations between ASD and comorbidities reviewed in this paper (dotted arrow). The DAG helps identify new pathways mediated by dysregulated phosphate metabolism for future research in the cause and prevention of ASD and associated comorbidities.

Figure 3. Dysregulated phosphate metabolism is causatively linked to both autism spectrum disorder and comorbidities associated with autism spectrum disorder.
Conclusion
Evidence reviewed in this paper suggests that the effects of dysregulated phosphate metabolism and phosphate toxicity in ASD appear across the full lifespan, from the maternal stages of early development, throughout childhood and into adulthood. Dysregulated phosphate is proposed to stimulate gliosis of the brain in ASD, which disrupts neuro-circuitry by blocking axon extension and shortening neurite outgrowth. Children with ASD were found to have a wide variety of immune and neuroinflammatory responses. The rapid increase in ASD prevalence coincidences with the general population's increased consumption of ultraprocessed foods that are high in phosphate additives. Dysregulated phosphate metabolism is also causatively linked to comorbidities associated with ASD such as abnormal proliferation of cells, Akt kinase cell signalling and PTEN inhibition in cancer and tuberous sclerosis. Comorbidities also include calcium phosphate precipitation in mitochondrial dysfunction, impaired hormone secretion in diabetes, seizures in epilepsy, inflammatory and immune responses in CKD, obesity linked to excessive intake of ultraprocessed food, hyperphosphorylation in tauopathy, atrial fibrillation and heart failure in CVD, and bone resorption and ectopic calcification in bone mineral disorders.
Although other environmental factors and toxic conditions besides phosphate toxicity may contribute to ASD, findings implicating dysregulated phosphate metabolism in ASD warrant further investigation. The evidence presented in the present paper offers novel insights and directions for future research linking dysregulated phosphate metabolism with the aetiology of ASD. Additionally, future studies examining the effect of reduced dietary phosphorus in ASD are warranted.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The author declares no conflict of interest.







