1. Introduction
Hallucinations are classically defined as perception-like experiences that occur without an external stimulus, in an awake state, with the physical properties and the sense of reality of real perceptions, and that are not under voluntary control [Reference Aleman and de Haan1, Reference Slade and Bentall2]. Hallucinations may involve all sensory modalities but, in patients with schizophrenia, their prevalence differs according to the sensory modality involved. Specifically, their lifetime prevalence is estimated at 64–80% for auditory, 23–31% for visual, 9–19% for tactile, and 6–10% for olfactory hallucinations [Reference McCarthy-Jones, Smailes, Corvin, Gill, Morris and Dinan3]. Given their predominance, hallucinations in the auditory modality (AH) are considered a hallmark of schizophrenia and have been the focus of most studies in the field. By contrast, relatively little consideration has been given to the co-occurrence of hallucinations in other modalities. Recently, McCarthy-Jones et al. reported that 30–37% of patients with lifetime AH had also experienced visual hallucinations (VH), and that, in reverse, 83–97% of patients with experience of VH had also experienced AH [Reference McCarthy-Jones, Smailes, Corvin, Gill, Morris and Dinan3]. This co-occurrence suggests common hallucinatory mechanisms in addition to specific modality-dependent dysfunctions [Reference Waters, Collerton, ffytche, Jardri, Pins and Dudley4]. Critically, the presence of VH in addition to AH (V + AH) is associated with a more severe psychopathological profile and a less favorable prognosis [Reference Clark, Waters, Vatskalis and Jablensky5]. Due to their impact on functioning, there is a crucial need to increase knowledge about these symptoms and particularly their underlying cognitive mechanisms.
During the last decades, some cognitive models have been proposed to explain positive symptoms such as hallucinations [Reference Waters, Allen, Aleman, Fernyhough, Woodward and Badcock6]. One of them assumes that hallucinations arise from a failure of reality-monitoring processes, specifically resulting in misattribution of internally generated events, such as thoughts or mental images, as being perceived from an external source [Reference Bentall, Baker and Havers7–Reference Morrison, Wells and Nothard9]. This type of misattribution, also called an externalization bias, may result from mental imagery having more perceptual characteristics than expected or from reduced cognitive operations associated with imagined information making the memory unlikely to have been internally-generated [Reference Johnson and Raye10, Reference Johnson, Hashtroudi and Lindsay11]. Several studies and meta-analyses have supported this model by showing that patients with hallucinations have a greater tendency to misattribute internal items to external sources than patients without hallucinations and healthy individuals [Reference Brookwell, Bentall and Varese12, Reference Brunelin, Combris, Poulet, Kallel, D’Amato and Dalery13]. Even if early reality-monitoring models of hallucinations [Reference Bentall, Baker and Havers7] did not propose that the deficits would concern some specific sensory modalities of hallucinations rather than others, most of the studies focused on AH. For instance, we reported that patients with AH misattributed more words they had imagined as being actually heard compared to patients without AH [Reference Brunelin, Combris, Poulet, Kallel, D’Amato and Dalery13]. To the best of our knowledge, only few studies investigated reality-monitoring deficits in relationship with hallucinations in the visual modality in patients with schizophrenia. They showed that patients with VH misremembered items that had been presented as words as having been presented as pictures [Reference Aynsworth, Nemat, Collerton, Smailes and Dudley14–Reference Stephan-Otto, Siddi, Senior, Cuevas-Esteban, Cambra-Martí and Ochoa16]. These results suggest that a failure in reality-monitoring processes may constitute a good candidate for the mechanism underlying hallucinatory phenomenon. However, little is known about the impact of presenting hallucinations in both visual and auditory modalities on reality-monitoring performance.
In this context, the aim of the present study was to test whether reality-monitoring deficits are associated with V + AH in schizophrenia. To this end, we investigated reality-monitoring performances in two groups of schizophrenia patients with hallucinations: a group with only AH (i.e., patients that never reported VH) and a group with V + AH, as done in studies exploring VH in schizophrenia [Reference Amad, Cachia, Gorwood, Pins, Delmaire and Rolland17–Reference Rolland, Amad, Poulet, Bordet, Vignaud and Bation19]. We hypothesized that patients with V + AH would display more severe reality-monitoring deficits than patients with AH only. More precisely, we proposed that experiencing hallucinations in several sensory modalities would increase the perceptual characteristics associated with imagined events and lead to a more severe externalization bias.
2. Materials and methods
2.1 Participants
Fifty patients with schizophrenia were included in the study. All participants met the DSM-IV-TR criteria for schizophrenia based on a clinical interview with an experienced psychiatrist using the Mini-International Neuropsychiatric Interview (M.I.N.I) [Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller20]. All participants were native French-speakers and displayed refractory treatment-resistant AH, defined as the persistence of daily AH despite antipsychotic treatment at an adequate dosage for at least 6 weeks. The Positive and Negative Syndrome Scale (PANSS) was used to evaluate the general symptomatology [Reference Kay, Fiszbein and Opler21] and the Auditory Hallucination Rating Scale (AHRS, range 0–42) was used to provide a specific and extensive measure of AH severity [Reference Hoffman, Hawkins, Gueorguieva, Boutros, Rachid and Carroll22]. Participants were carefully divided into two groups based on the presence of VH measured by the item #6 of the Scale for the Assessment of Positive Symptoms (SAPS [Reference Andreasen23]): a group of patients with AH and no VH (AH group, n = 24, SAPS-item #6 = 0) and a group of patients with AH and VH (V + AH group, n = 22, SAPS-item #6 ≥ 2, i.e., VH ranging from “2-mild” to “5-severe” intensity). SAPS-item #6 assess VH by asking if “the patient sees shapes or people that are not actually present”. Participants with questionable VH (SAPS-item #6 = 1) were excluded (n = 4). Participants characteristics are detailed in Table 1.
Table 1 Demographic and clinical characteristics of patients with AH and patients with V + AH.

* All values are mean (SD). Groups were compared using two-sample t-tests, except for gender where a Chi-square test was used.
* significant difference between groups (p < 0.05).
All patients gave their written informed consent. All experiments were approved by a local ethics committee and performed in accordance with relevant guidelines and regulations.
2.2 Reality-monitoring task
Reality-monitoring performances were assessed using a previously described task that measures confusion between imagined words and heard words (‘Hear-Imagine’ task) [Reference Brunelin, Combris, Poulet, Kallel, D’Amato and Dalery13, Reference Brunelin, d’Amato, Brun, Bediou, Kallel and Senn24]. All participants completed a short practice trial to become acquainted with the task requirements and to ensure the understanding and the good realization of instructions. The task was separated into two phases: an encoding phase and a memory retrieval phase. During the encoding phase, 16 words were presented one by one in a randomized order in the centre of a computer screen for 3 s. Each word was immediately preceded by its respective instruction, either “Imagine yourself hearing the following word” or “Listen to the following word”. Half of the words (8 words) had to be heard and their visual presentation was accompanied by an auditory stimulus (i.e., the experimenter voice speaking the word once). The other half (8 words) had to be imagined by the participant and no auditory stimulus was presented. The memory retrieval phase began immediately after the end of the encoding phase. During this phase, participants were presented with a response grid including the 16 words previously presented during the encoding phase, plus 8 new words which had not previously been presented. For each word, participants were asked to answer whether the word was heard, imagined or if the word was not presented during the encoding phase.
Two main outcomes were measured: 1) the externalization bias, measured as the number of imagined words that were classified as heard (range 0–8), and 2) the internalization bias, measured as the number of heard words that were classified as imagined (range 0–8) [Reference Brunelin, Combris, Poulet, Kallel, D’Amato and Dalery13].
2.3 Statistical analyses
Data were analyzed with JASP (Version 0.9; JASP Team, 2018). Between group comparisons were computed using two-sample t-tests for continuous outcomes and Chi-square tests for categorical data. For misattribution biases comparisons, Cohen's d effect sizes were also computed. All tests were two-tailed and statistical significance was set at p < 0.05. In case of a significant effect, the potential confounding role of gender was assessed using a two-way ANOVA with Group and Gender as between-group factors.
3. Results
3.1 Demographic and clinical characteristics
No significant differences were reported between AH and V + AH groups for age, education, illness duration, AH severity measured by the AHRS and general symptomatology severity assessed by the total PANSS score (all p > 0.05, see Table 1). However, the two groups differed significantly on gender (χ2 = 8.626, p = 0.003).
3.2 Reality monitoring performance
Patients with V + AH displayed a significantly higher externalization bias as compared to patients with AH (t(44) = -2.78, p = 0.008, d = -0.82; see Fig. 1). In other words, patients with V + AH misrecognized more imagined words as being heard (M = 2.18, SD = 1.71) than patients with AH only (M = 1.00, SD = 1.14). No difference was found between groups for the internalization bias, i.e. the misrecognition of heard words as being imagined (t(44) = 1.04, p = 0.31, d = 0.31).
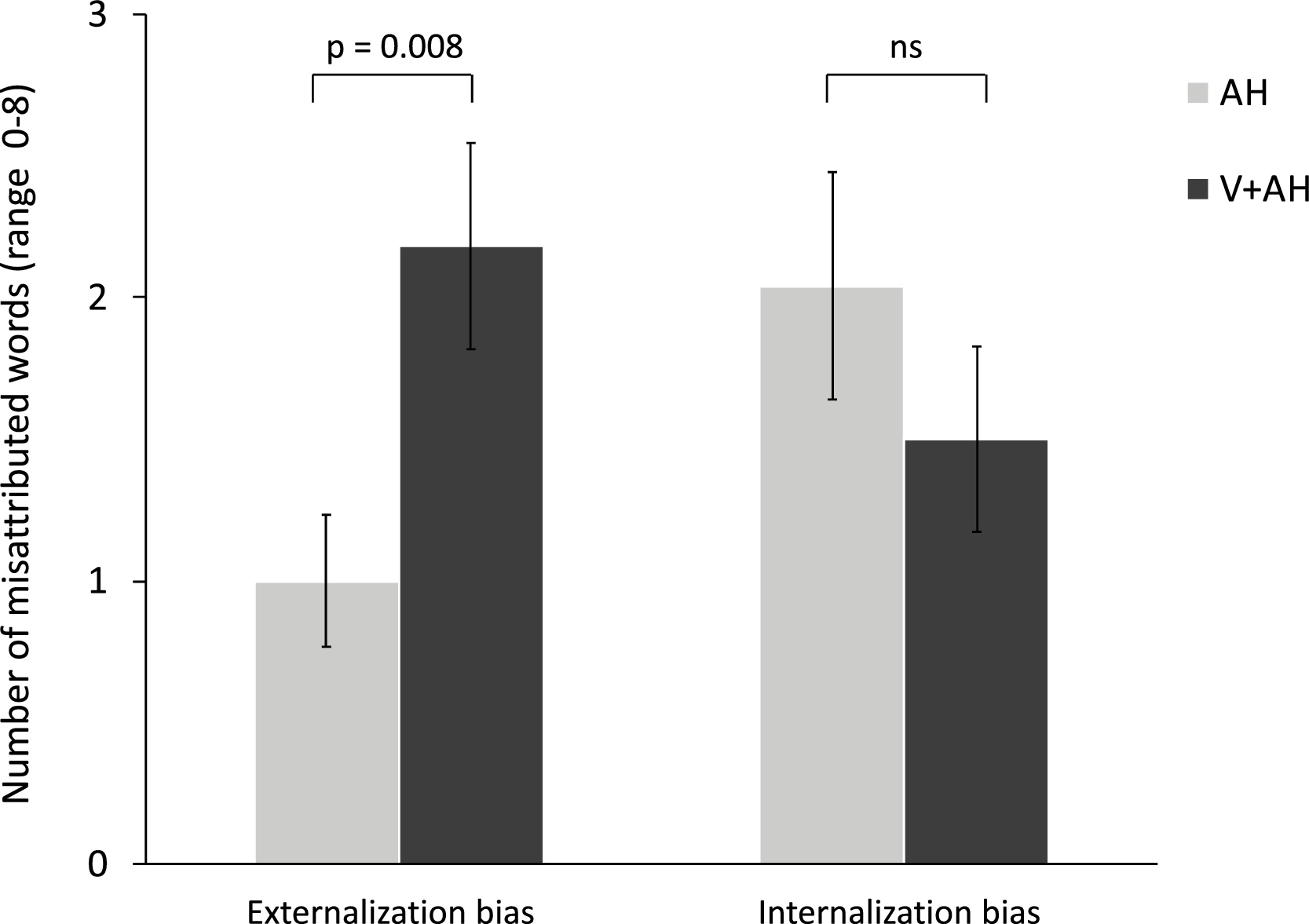
Fig. 1. Comparison of reality-monitoring performance between patients with V + AH (dark grey bars) and patients with AH only (light grey bars). The externalization bias is measured as the number of imagined words that were classified as heard (range 0–8) and the internalization bias is measured as the number of heard words that were classified as imagined (range 0–8). [Reference Brunelin, Combris, Poulet, Kallel, D’Amato and Dalery13] Results are displayed as mean +/- standard error. ns = not significant.
In order to rule out the potential confounding role of gender in our analyses, we examined the effect of gender on the externalization bias with a two-way ANOVA with Group and Gender as between-group factors. We found no significant effects of Gender (F(1, 42) = 0.31, p = 0.58) and Group*Gender interaction (F (1, 42) = 1.75, p = 0.20). By contrast, the effect of Group remained significant (F(1, 42) = 4.70, p = 0.036).
4. Discussion
The present study proposed an original investigation of reality-monitoring performance in patients with schizophrenia who present with V + AH. Findings indicated that patients with V + AH displayed a higher externalization bias than patients with AH, which means that patients with V + AH were more likely to remember imagined words as being perceived compared to patients with AH only.
The existence of a misattribution bias in patients with VH has already been described in the literature in schizophrenia [Reference Aynsworth, Nemat, Collerton, Smailes and Dudley14–Reference Stephan-Otto, Siddi, Senior, Cuevas-Esteban, Cambra-Martí and Ochoa16, Reference Brébion, Ohlsen, Bressan and David25]. For instance, Brébion et al. (2008) found that patients with VH were more likely to report that they had seen pictures of items when only the verbal label of these items had been displayed. However, in these studies, reality-monitoring was tested using stimuli in the visual modality only (i.e. words and pictures), making it difficult to decipher whether the externalization bias was modality-specific or not. By contrast, Arguedas et al.' s investigated the modality-specificity of reality-monitoring impairments [Reference Arguedas, Stevenson and Langdon26]. Authors found that patients with olfactory hallucinations were less accurate in distinguishing between imagined and smelled odors than patients with AH and healthy controls and, additionally, that patients with AH were less accurate in distinguishing the source of a spoken word. However, the verbal reality-monitoring task used in Arguedas et al.' s design did not involve a distinction between imagined and perceived events, as done in our study, but a distinction between self-produced words and words produced by the experimenter. It is likely that such distinction is not related to the same cognitive mechanisms as those involved in discriminating imagination from perception.
In our study, we tested reality-monitoring performances in the auditory modality and demonstrated that V + AH patients were more likely to misattribute imagined words as being heard than patients with AH only. Given that the two groups did not differ in AH and other symptoms severity, we propose that the misattributions observed may not be specific of the sensory modality but rather reflect an amodal deficit in discriminating imagination from reality. This interpretation is consistent with Garrison et al.’ study, which reported that even if reality-monitoring accuracy was better for imagined stimuli in the visual than in the auditory modality, the level of externalization bias was independent of the modality of presentation of the stimuli [Reference Garrison, Bond, Gibbard, Johnson and Simons27]. To confirm that the modality used to measure reality-monitoring did not influence the observed externalization bias, it would be of great interest to include a visual reality-monitoring task, together with the present auditory task, in a further study.
One can hypothesize that patients with V + AH display a more severe misattribution bias because of increased perceptual details associated to the imagined event that may lead to greater confusion when identifying the source of this event. This is consistent with the hypothesis of increased vividness of mental images associated with VH, which has been supported by evidence of a relationship between proneness to VH and vivid imagery in nonclinical people with VH [Reference Aynsworth, Nemat, Collerton, Smailes and Dudley14]. In addition, the hypothesis of increased perceptual details associated to the imagined event is consistent with the abnormal overactivity of sensory cortice observed during hallucinations such as the hyperactivation of superior temporal regions for AH and visual cortices for VH [Reference Zmigrod, Garrison, Carr and Simons28], which might produce more vivid perceptual content than usually associated with imagined events. Alternative explanations for the increased externalization bias independently of the vividness of mental images would be that patients with V + AH associate less cognitive operations information to imagined events [Reference Johnson29] or display greater deficits in the ability to compare imagined and real events [Reference Bentall and Slade30].
Besides, the findings of a misattribution bias in patients with VH is consistent with a previous study showing a greater propensity to report imagined stimuli as percepts in patients with Parkinson disease and VH [Reference Barnes, Boubert, Harris, Lee and David31]. Since a similar misattribution bias was reported in nonclinical people with high predisposition to VH, one could hypothesize that the reality-monitoring deficit constitutes a common mechanism that underlies VH regardless of the diagnostic category.
Despite the original findings, several limits should be acknowledged. First, one can hypothesize that the observed difference between groups is supported by the difference in gender proportion between our samples. Since our analyses revealed no effect of gender on the externalization bias, the between-group difference in terms of gender is unlikely to account for the present observation of an increased externalization bias in patients with V + AH. The observed gender imbalance between V + AH and AH patients is in line with some previous studies reporting a higher risk for VH in females than in males [Reference Thomas, Mathur, Gottesman, Nagpal, Nimgaonkar and Deshpande32]. However, a more recent study investigating the prevalence and co-occurrence of hallucinations across the auditory, visual, olfactory, and tactile modalities, in two large samples of people diagnosed with chronic schizophrenia-spectrum disorders found no consistent association between VH (or any modality of hallucination) and gender [Reference McCarthy-Jones, Smailes, Corvin, Gill, Morris and Dinan3]. Second, we cannot exclude that the more severe reality-monitoring deficits observed in the V + AH group could be due to more severe general cognitive deficits in V + AH. In the current sample, we compared PANSS cognitive dimension scores [Reference Lindenmayer, Bernstein-Hyman and Grochowski33] between groups and observed no differences between V + AH (mean = 11.73, SD = 3.24) and AH groups (mean = 11.77, SD = 3.28; p = 0.96). However, further studies with standardized cognitive evaluations are required to address this issue. In addition, the greater deficits observed in the V + AH group could be linked to greater visual processing deficits. Indeed, studies in patients with Parkinson disease have reported a relationship between VH and altered visual processing [Reference Diederich, Fénelon, Stebbins and Goetz34, Reference Bernardin, Schwitzer, Angioi-Duprez, Giersch, Jansen and Schwan35] and there is also evidence that patients with Parkinson disease and VH showed deficits in both visual perception and source monitoring [Reference Barnes, Boubert, Harris, Lee and David31]. To decipher on the contribution of visual abnormalities to the reality-monitoring deficits in patients with schizophrenia, studies investigating visual processing together with reality-monitoring are needed. Third, one potential limitation of the present study is that no healthy individuals were compared to the patient groups. However, regarding repeated evidence of reality-monitoring impairment in schizophrenia (reviewed in [Reference Brookwell, Bentall and Varese12]), we can reasonably assume that our samples of participants display reality-monitoring deficits. Furthermore, the study was designed to assess the effects that were specific to the sensory modality involved in hallucinations, i.e., AH or V + AH, rather than the effects that were more broadly related to schizophrenia and medication. Fourth, although the task used to measure reality-monitoring processes in our study was similar to the paradigms that have been previously used to assess reality-monitoring, with imagined and heard words presented visually [Reference Brunelin, Combris, Poulet, Kallel, D’Amato and Dalery13, Reference Keefe, Arnold, Bayen and Harvey36–Reference Moseley, Mitrenga, Ellison and Fernyhough38], this task came with some limitations. Indeed, we cannot exclude that participants may not have always followed the instruction to imagine hearing the visually presented words and that it could have affected their performance during the retrieval phase. Finally, the tools used to assess hallucinations in our sample of schizophrenia patients did not allow us to differenciate multimodal hallucinations in different sensory domains at the same time, as well as serial multimodal hallucinations that occurred at separate times. Since simultaneous multimodal hallucinations are associated with a greater sense of reality and distress [Reference Dudley, Aynsworth, Cheetham, McCarthy-Jones and Collerton39], it would have been relevant to explore whether they were associated to a higher externalization bias.
In summary, we present evidence that patients with schizophrenia who have V + AH display a higher deficit in reality-monitoring abilities than patients with schizophrenia who only have AH. Experiencing hallucinations in two sensory modalities is associated with a greater tendancy to misattribute imagined events as being actually perceived from an external source. A better understanding of the cognitive processes underlying the presence of V + AH in patients with schizophrenia could help identify some potential targets for clinical interventions. These findings support the interest of developping psychological interventions that aim to overcome reality-monitoring deficits in patients with V + AH, as done by two previous studies reporting promising results [Reference Favrod, Vianin, Pomini and Mast40, Reference Subramaniam, Luks, Fisher, Simpson, Nagarajan and Vinogradov41].
Acknowledgement
The authors thank Eliot Cochise Joutard and Caroline Damasceno for their help during data collection.





Comments
No Comments have been published for this article.