Introduction
Over the past two decades, the relationship between autism spectrum disorder (ASD) and catatonia has been investigated by numerous studies in the scientific literature. ASDs are neurodevelopmental disorders characterized by difficulties in social communication, restricted and repetitive behaviors or interests, and hyper/hyporesponsiveness to sensory stimuli. In addition to the diagnosis of overt ASD, great importance has been given in recent years to subthreshold autistic manifestations, widespread to varying degrees in the general population and investigated through dedicated assessment tools [Reference Dell’Osso, Gesi, Massimetti, Cremone, Barbuti and Maccariello1]. Catatonia, originally described in 1874 by Kahlbaum and long relegated to the realm of schizophrenia, is currently defined by the DSM-5-TR [2] as a severe neuropsychiatric syndrome characterized by three or more symptoms among catalepsy, waxy flexibility, stupor, muteness, negativism, agitation, posturing, stereotypes, mannerisms, grimacing, echolalia, and echopraxia. The DSM-5-TR trajectory appeared to be receptive to a less strict definition of the catatonic clinical picture and an expansion of its boundaries. It has been stressed that the lack of narrow boundaries may result in the inclusion of overly divergent conditions, like akathisia, under the shadow of catatonic symptoms [Reference Ungvari, Caroff and Gerevich3]. Although provocative, these critiques raise reflexive questions about the existence of something we might call a spectrum of catatonia that could recognize a wide range of etiologies and a wide range of manifestations with varying degrees of severity, which is somewhat consistent with the conceptualization of catatonia in DSM-5 as a trans-diagnostic specifier, potentially associated with every mental disorder, with a multitude of different combinations of symptoms, and denoting atypical and incomplete manifestations under the umbrella of catatonia not otherwise specified. Prominent among the mental disorders associated with catatonia are neurodevelopmental disorders and, among them, ASD. The complication of ASD with catatonia is not an infrequent occurrence, as already observed by Lorna Wing’s studies of autism [Reference Wing and Shah4]. A recent systematic review [Reference Vaquerizo-Serrano, Salazar De Pablo, Singh and Santosh5] showed that 10.4% of individuals affected by ASD have catatonia, highlighting a clinical overlap between the two disorders for which several explanations have been proposed: a common alteration in the GABAergic system [Reference Northoff, Steinke, Czcervenka, Krause, Ulrich and Danos6], in neural circuits [Reference Dhossche, Carroll and Carroll7] or in the size of cerebellar structures [Reference Courchesne, Yeung-Courchesne, Hesselink and Jernigan8], as well as a possible genetic connection, derived from susceptibility regions on chromosome 15 [Reference Chagnon9].
In two prevalence studies [Reference Wing and Shah4, Reference Billstedt, Gillberg and Gillberg10], catatonia was found to occur in 12–17% of a large sample of adolescents and young adults with ASD. Numerous clinical features are shared by ASD and catatonia, such as mutism and echolalia, stereotyped movements and repetitive behaviors, and negativism and arousal. This clinical overlap could be responsible, on one hand, for a propensity to overestimate subthreshold catatonia among autistic subjects and, on the other, for a failure to recognize catatonic symptoms that first appear in patients with ASD [Reference Dell’Osso, Toschi, Amatori and Gesi11]. Indeed, although anecdotal, previous descriptions have indicated that catatonia may slowly develop over the course of autism, often preceded by isolated manifestations and a slow deterioration in functioning, until it assumes a chronic character [Reference Kakooza-Mwesige, Wachtel and Dhossche12]. One of the few systematic studies on catatonia among young people with ASD found that the largest percentage of autism-related cases occur among individuals with Asperger’s disorder (AD), rather than among classic or atypical autistic patients [Reference Kakooza-Mwesige, Wachtel and Dhossche12]. As it is known that a significant percentage of patients with AD remain undiagnosed throughout adolescence and adulthood [Reference Dell’Osso, Lorenzi and Carpita13], it would be reasonable to consider the possibility that they may develop catatonia at some point in their lives. Such a scenario would require, from clinicians working with adults, who are often unfamiliar with the diagnosis of ASD, an ability to untangle the complex clinical picture of such patients and to identify appropriate treatment.
Moreover, growing literature supports the existence of an autistic subthreshold phenotype, free from significant impairment in daily living but phenomenologically and etiologically in continuity with the clinical spectrum of autistic disorders. Such subthreshold autism spectrum, although unrecognized, could increase the risk of catatonia. Since it is believed that the autistic dimension is not only present in individuals with ASD, but also widespread in the general population as a broader autistic phenotype or autistic traits [Reference Dell’Osso, Gesi, Massimetti, Cremone, Barbuti and Maccariello1], we might speculate that catatonia might be related to this autistic dimension even when not clinically evident and, as discussed in recent work, especially in patients with a history of traumatic events [Reference Dell’Osso, Amatori, Gesi and Carmassi14]. Beyond the recurrent speculations regarding the conceptualization of autism as an early form of catatonia, some studies conducted on a validated animal model of autism have shown the beneficial effect of pharmacologically induced seizures and electroconvulsive therapy, one of the most effective treatments available for catatonia, in reducing autism-like behaviors in mice [Reference Hagen, Shprung, Minakova, Washington, Kumar and Shin15]. In addition, several studies on the pathophysiology of catatonia and autism suggest the existence of a common phenomenon of GABAergic cortical dysregulation resulting in an imbalance between arousal and inhibition in the CNS and a failure to cognitively control emotions [Reference Northoff, Kötter, Baumgart, Danos, Boeker and Kaulisch16, Reference Ellul and Choucha17]. In parallel, candidate loci for autism and catatonia have been identified on the long arm of chromosome 15 [Reference Cook, Lindgren, Leventhal, Courchesne, Lincoln and Shulman18–Reference Stöber, Seelow, Rüschendorf, Ekici, Beckmann and Reis20].
The aim of this study is to investigate the relationship between autism traits, signs, symptoms, and manifestations, and catatonic spectrum and to determine which among the autism spectrum features are the most related to the catatonic spectrum.
Materials and Methods
Data have been collected between November 2021 and January 2022 at six Italian University Departments of Psychiatry, coordinated by the University of Pisa: University of Campania “Luigi Vanvitelli,” University of Pavia, University of Messina, La Sapienza University of Rome, and University of Brescia.
Study sample and procedures
The total sample consisted of 376 subjects distributed in four diagnostic groups, all evaluated according to DSM-5 diagnostic criteria. Exclusion criteria were: age under 18 years, language or intellectual impairment affecting the possibility to fulfill the assessments, mental disability, poor cooperation skills, and ongoing psychotic symptoms. Specifically, the four groups were individuated as follows: 86 subjects endorsing at least three symptom criteria for catatonia (CTN); 81 subjects diagnosed with borderline personality disorder (BPD); 104 subjects diagnosed with major depressive disorder (MDD); 105 healthy control individuals without current or lifetime mental disorders (HC) and belonging to health care and paramedical personnel. All subjects were aged 18–60 years old and signed a written informed consent. The Structured Clinical Interview for DSM-5, Research Version (SCID-5-RV) [Reference First, Williams, Karg and Spitzer21] was used to confirm the diagnoses of BPD and MDD, as well as the absence of mental disorders among CTL. The study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee of the Azienda Ospedaliero-Universitaria of Pisa approved all recruitment and assessment procedures. Eligible subjects provided written informed consent, after receiving a complete description of the study and having the opportunity to ask questions. Subjects were not paid for their participation according to Italian legislation.
Measures
Assessment procedures included the SCID-5-RV [Reference First, Williams, Karg and Spitzer21], the Adult Autism Subthreshold Spectrum (AdAS Spectrum), and the Catatonia Spectrum (CS). Questionnaire were carried out by psychiatrists who were trained and certified in the use of the study instruments.
The Adult Autism Subthreshold Spectrum
AdAS Spectrum is a questionnaire devised to assess not only full-blown ASD, but also the broader spectrum of subthreshold autism, in subjects with normal intelligence and without language impairment across the lifetime. It allows evaluating a wide area of clinical and nonclinical traits, typical and atypical manifestations, including some gender-specific features. The instrument is composed of dichotomous questions, grouped into seven domains: childhood/adolescence, verbal communication, nonverbal communication, empathy, inflexibility and adherence to routine, restricted interests and rumination, and hyper–hypo reactivity to sensory input. In the validation study [Reference Dell’Osso, Gesi, Massimetti, Cremone, Barbuti and Maccariello1], the AdAS Spectrum questionnaire demonstrated an excellent reliability and a strong convergent validity with other scales employed in this field, such as the Autism-Spectrum Quotient Test [Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley22] and the e Ritvo Autism and Asperger Diagnostic Scale 14-item version [Reference Eriksson, Andersen and Bejerot23]. The AdAS Spectrum has been used, in recent years, within numerous studies focusing on the autism spectrum both in clinical and in nonclinical settings [Reference Carpita, Cremone, Amatori, Cappelli, Salerni and Massimetti24–Reference Dell’Osso, Cremone, Amatori, Cappelli, Cuomo and Barlati33].
The Catatonia Spectrum
CS is a self-assessment questionnaire that investigates nuclear, subthreshold, atypical, and partial manifestations of the CS, referred to across the lifespan, divided into domains, and explored with a set of questions. The CS consists of 74 items and is divided into eight domains: (a) psychomotor activity (stupor); (b) verbal response (mutism); (c) repetitive movements (stereotypes); (d) artificial expressions and actions (mannerisms); (e) oppositivity or poor response to stimuli (negativism); (f) response to instructions given from outside (automatic obedience); (g) automatisms; (h) impulsivity. For each item, there is a dichotomous answer “Yes” and “No.”
In the validation study [Reference Dell’Osso, Amatori, Cappelli, Cremone, Massimetti and Gravina34], the CS questionnaire demonstrated excellent internal consistency and test–retest reliability and strong convergent validity with alternative dimensional measures of catatonia, such as the Bush-Francis Catatonia Rating Scale and the Bush-Francis Catatonia Screening Instrument [Reference Bush, Fink, Petrides, Dowling, Francis and Catatonia.35].
Statistical analysis
Analyses were performed using Statistical Package for the Social Sciences (SPSS) version 26.0 [36].
Relationships between AdAS Spectrum and CS total scores within the total sample, gender and diagnostic subgroups were studied by calculating Pearson’s correlation coefficients and performing linear regression analyses.
Multiple linear regression was used to identify the AdAS Spectrum domains most strongly correlated with the total CS score.
To study catatonic symptoms in groups with different levels of autism severity, we divided the sample on the basis of the two cut-off points provided by the AdAS Spectrum on its total score [Reference Dell’Osso, Carmassi, Cremone, Muti, Salerni and Barberi37], obtaining three subgroups: participants with ASD (ASD group); participants with subthreshold autistic traits (AT group); and participants without autistic traits (Non-AT group) and comparing the three obtained groups on CS total score within each the four diagnostic subgroups; for this purpose, we used a one-way analysis of variance (ANOVA) followed by Bonferroni’s test for post hoc comparisons. Finally, to complete the study of the relationship between the three ASD, AT, and Non-AT groups and the four diagnostic groups, contingency tables and the Chi-Square test were used.
Results
The four diagnostic groups were sufficiently homogeneous in terms of age and gender.
The catatonia group included subjects with a mean age of 40.45 years (±11.85) and consisted of 34 (39.5%) males and 52 (60.5%) females. The MDD subjects had a mean age of 40.74 (±11.46) years and consisted of 36 (34.6%) males and 68 (56.7%) females. The group of BDP subjects had a mean age of 40.54 (±13.599) years and consisted of 31 (38.3%) males and 50 (61.7%) females. The group of HC subjects had a mean age of 37.71 (±11.02) years and consisted of 38 (36.2%) males and 67 (63.8%) females.
A statistically significant positive correlation was found between AdAS Spectrum and CS total score within the total sample (r = 0.762; p < 0.001), the gender subgroups (r = 0.740; p < 0.001 in males; r = 0.776; p < 0.001 in females) and in the diagnostic categories (Table 1).
Table 1. Correlations between total Adult Autism Subthreshold (AdAS) and Catatonia Spectrum (CS) scores in the diagnostic groups.

Abbreviations: BPD, borderline personality disorder; HC, helthy control; MDD, major depressive disorder; CTN, catatonia.
Table 2 reports the regression coefficients and their confidence intervals obtained considering total AdAS Spectrum score and CS total score, respectively, as independent and dependent variables in seven regression analyses conducted within the total sample, the male and female gender subgroups, and the four diagnostic groups. All the regression coefficients appeared to be significant and positive underlining a strong general relation between autistic and catatonic symptomatology.
Table 2. Seven simple linear regressions [dependent variable: Catatonia Spectrum (CS) total score; predictors: total Adult Autism Subthreshold (AdAS) Spectrum score] related to seven different subgroups, with related regression coefficients (B) and standard error (SE), significance (p), and confidence interval (CI).
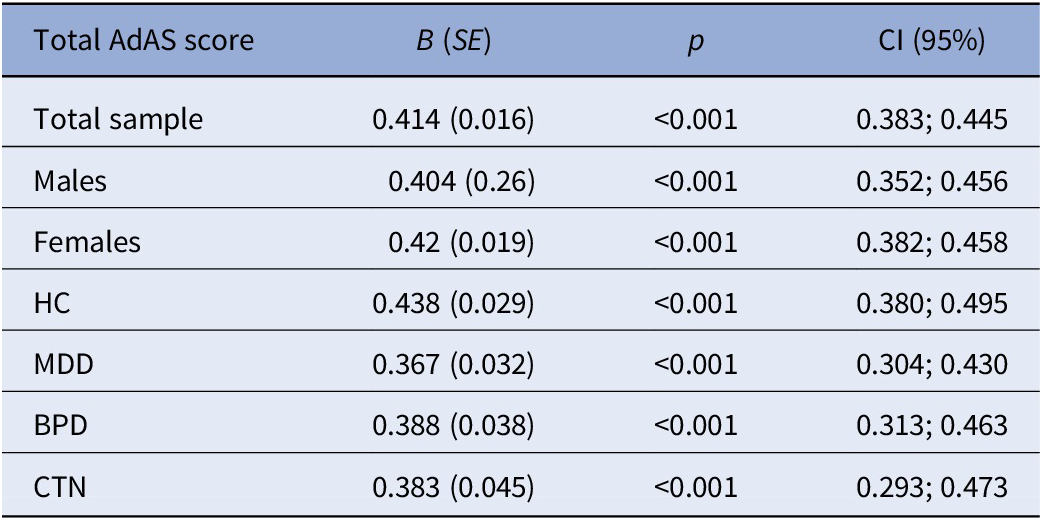
Abbreviations: BPD, borderline personality disorder; HC, healthy control; MDD, major depressive disorder; CTN, catatonia.
In order to get a clearer view of the correlation between total AdAS Spectrum score and total CS score within the different diagnostic groups, we constructed a scatter graph in which the differently colored plotter points represent the subjects belonging to the four diagnostic subgroups (Figure 1).

Figure 1. Scatter graph showing the correlation between Adult Autism Subthreshold (AdAS) Spectrum and Catatonia Spectrum (CS) total score within the four diagnostic groups, represented by the plotter points.
Table 3 reports the results of the multiple linear regression utilizing the total CS score as a dependent variable and the AdAS Spectrum domain scores as an independent variable. The AdAS Spectrum domains found to be significantly and strongly correlated with the total CS score were: hyper–hypo reactivity to sensory input, verbal communication, nonverbal communication, restricted interests and rumination, and inflexibility and adherence to routine.
Table 3. Multiple linear regression [dependent variable: total Catatonia Spectrum (CS) score; independent variables: Adult Autism Subthreshold (AdAS) Spectrum domain scores] with related regression coefficients (B) and standard error (SE), significance (p), and confidence interval (CI).

Note: Adjusted R 2 = 0.589.
p value < 0,05
The ASD, AT, and Non-AT groups were found to be distributed across all diagnostic groups, and in particular, the ASD group was most represented in patients with BPD (56.5%) and in second place in patients with Catatonia (31.1%), the AT group in patients with Catatonia (31.1%), and the non-AT group in healthy controls (75.6%).
Table 4 shows the results of the ANOVA comparing the three autism severity groups based on the CS total score within each diagnostic subgroup. The CS score increased significantly from the non-AT group to the AT group to the ASD group. In other words, the greater the severity of autism, thus the higher the AdAS Spectrum score, the higher the CS score. See also Figure 2 for greater clarity.
Table 4. Comparison of the three autistic severity groups based on the Catatonia Spectrum (CS) total score within each diagnostic subgroups.

Abbreviations: ASD, autism spectrum disorder; AT, autistic traits; Non-AT, non autistic traits; BPD, borderline personality disorder; HC, healthy control; MDD, major depressive disorder; CTN, catatonia.

Figure 2. Comparison of the three autism severity groups based on the total Catatonia Spectrum (CS) score. Each of the three lines represents an autism severity group. The points on each line correspond to the average total CS scores in the four diagnostic groups.
Discussion
The results of the present study suggest a strong correlation between autism spectrum (autistic traits and ASD signs, symptoms, and behavioral manifestation) and catatonic spectrum (nuclear, subthreshold, atypical, and partial manifestations), in agreement with previous literature regarding the correlation between ASD and catatonia.
The finding of higher CS scores as autism severity increases supports the hypothesis that catatonia could be considered as a neurodevelopmental disorder, in particular a late and potentially preventable form of autism [Reference Northoff, Steinke, Czcervenka, Krause, Ulrich and Danos6, Reference Chagnon9]. In this regard, a recent study reported the case of a young child in whom the onset of catatonia, occurring after trauma and against the backdrop of a stressful family environment, led to the diagnosis of an underlying ASD [Reference Quilliam, Quilliam, Turnbull, Parkinson and Oligbu38].
The autism spectrum features that, in the present study, were found to be most correlated with catatonic spectrum manifestations are, in order of importance, hypo/hypersensitivity to sensorial stimuli, difficulties in verbal and nonverbal communication, mental rumination, and repetitive behaviors. This finding could be explained by considering the role of trauma in the psychopathological trajectory originating from an autism spectrum-derived vulnerability and culminating in catatonic spectrum manifestations.
With regard to the phenomenology of catatonia, Shorter and Fink, in their book The Madness of Fear [Reference Shorter and Fink39], used a historical analysis to illustrate that catatonia has often been linked to and can be driven by fear. Acute emotional states, especially fear, have long been suggested as a crucial component of catatonia. A recent study reported that half of the patients with phenomenological descriptions reported significant distress at the time of catatonia, and more than one-third of patients explicitly reported fear [Reference Dawkins, Cruden-Smith, Carter, Amad, Zandi and Lewis40]. The role of trauma in influencing a vulnerable individual to develop catatonic symptoms was explored by a recent study, in which a meaningful correlation was found between the Bush-Francis Catatonia Scale and the Adverse Childhood Experience questionnaire [Reference Hortenstine and Youssef41]. Based on the same study, there appear to be two constructs of conditioning that predispose a subject to catatonic symptoms in response to traumatic events: external conditioning (e.g., the external environment) and internal conditioning (e.g., flashbacks or nightmares after a traumatic experience). In relation to vulnerability to trauma, clinical and scientific data show an increased risk of adverse events and trauma in individuals with ASD. It has been proposed that individuals with ASD may be more vulnerable to traumatic stress reactions due to difficulties with language comprehension, information processing, emotion regulation impairments, and increased levels of social isolation [Reference Mansell, Sobsey and Moskal42, Reference Brenner, Pan, Mazefsky, Smith and Gabriels43]. In addition, aspects of cognitive processes correlated with ASD, such as repetitive and perseverative tendencies, could contribute to the experience of trauma. In brief, if an experience appears to be particularly salient and perhaps distressing, such as a traumatic event, individuals with ASD may persevere in thoughts and feelings related to that experience, risking re-experiencing the past trauma over and over again [Reference Peterson, Earl, Fox, Ma, Haidar and Pepper44]. Finally, it has been proposed that the core symptoms of ASD may lead to chronic exposure to daily ASD-related stressors from sensory sensitivity and aversions [Reference Kerns, Newschaffer and Berkowitz45, Reference Wood and Gadow46]. In this context, the hypersensitivity typical of autistic individuals could be responsible for an increased intensity of post-traumatic re-experiencing, which is known to be associated with an altered processing of sensations from the external and internal world [Reference Harricharan, McKinnon and Lanius47]. This perspective would be in agreement with the so-called “Intense World Theory” according to which, in individuals with autism are characterized by hyper-perception, hyper-attention, hyper-memory, and hyper-emotionality. The progression of the disorder would be driven by over-reactions to experiences that lead the brain to a state of hyper-preference and over-selectivity, which becomes more extreme with each new experience and can be especially accelerated by particular emotional experiences and trauma [Reference Markram, Rinaldi and Markram48]. Considering these issues, the correlation between autistic traits and catatonic manifestations could be mediated by the vulnerability to trauma derived from particular dimensions of the autistic spectrum, such as mental rumination, communication impairment, and, especially, altered sensitivity to external and internal sensorial stimuli.
Conclusions
The results of the present study show a strong correlation between the autism spectrum (autistic traits and symptoms of ASD) and the manifestations of CS.
The autism spectrum domains found to be most closely related to CS are, in order of importance, hyper–hypo reactivity to sensory input, verbal communication, nonverbal communication, restricted interests and rumination, and inflexibility and adherence to routine.
Data Availability Statement
The data that support the findings are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the participants for their continued support and participation in the study.
Author Contributions
Conceptualization: L.D’O., G.A.; Data curation: L.D’O., G.A., B.N., D.G., F.B., C.B., M.L., I.B., N.B., M.D.G., G.D., M.N., A.R., M.R.A.M., M.P., P.P., A.V., C.C.; Formal analysis: G.M.; Investigation: L.D’O., G.A., B.N., D.G., F.B., C.B., M.L., I.B., N.B., M.D.G., G.D., M.N., A.R., M.R.A.M., M.P., P.P., A.V., C.C.; Methodology: L.D’O., G.A.; Project administration: L.D’O., G.A.; Supervision: L.D’O., C.C.; Writing—original draft: G.A.; Writing—review and editing: L.D’O., C.C.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Disclosure
The authors have nothing to disclose.









Comments
No Comments have been published for this article.