1 Introduction
Tic disorder is a neuropsychiatric condition characterized by short-lasting, sudden, habitual, non-rhythmic muscle contraction (motor tics) or vocalization (phonic tics) Reference American Psychiatric Association[1]. Tourette syndrome (TS) is diagnosed when both motor and phonic tics are present for at least one year Reference American Psychiatric Association[1]. The overall international prevalence of TS is approximately 1% Reference Robertson, Eapen and Cavanna[2]. The onset of tics is usually between 4 and 6 years of age, with peak severity occurring between ages 10 and 12 years Reference American Psychiatric Association[1]. In the majority of children with TS, symptoms generally diminish or disappear as adults, but a small percentage (approximately a third) will have persistent symptoms that require clinical attention [Reference American Psychiatric Association1, Reference Roessner, Plessen, Rothenberger, Ludolph, Rizzo, Skov, Strand, Stern, Termine, Hoekstra and ESSTS Guidelines Group3].
Several neural mechanisms have been proposed for TS. The observation that antipsychotics (i.e., agents that block dopamine D2 receptors) have been effective in treating tics has led to a hypothesis that heightened dopaminergic activity may be involved in TS Reference Roessner, Plessen, Rothenberger, Ludolph, Rizzo, Skov, Strand, Stern, Termine, Hoekstra and ESSTS Guidelines Group[3]. Studies using positron emission tomography further demonstrated elevated striatal dopamine release in individuals with TS [Reference Wong, Brasić, Singer, Schretlen, Kuwabara, Zhou, Nandi, Maris, Alexander, Ye, Rousset, Kumar, Szabo, Gjedde and Grace4, Reference Denys, de Vries, Cath, Figee, Vulink, Veltman, van der Doef, Boellaard, Westenberg, van Balkom, Lammertsma and van Berckel5]. In addition, alpha2-adrenergic agonists (e.g., clonidine) that inhibit the release of norepinephrine have also been effective anti-tic medications Reference Roessner, Plessen, Rothenberger, Ludolph, Rizzo, Skov, Strand, Stern, Termine, Hoekstra and ESSTS Guidelines Group[3], suggesting heightened central noradrenergic activity in TS Reference Hawksley, Cavanna and Nagai[6]. Studies that examined cerebrospinal fluid biogenic amines further demonstrated elevated levels of norepinephrine in TS patients compared with healthy individuals Reference Leckman, Goodman, Anderson, Riddle, Chappell, McSwiggan-Hardin, McDougle, Scahill, Ort, Pauls, Cohen and Price[7]. Moreover, stress-related neurobiological mechanisms seem to play a role, as evidenced by elevated cerebrospinal fluid levels of corticotropin-releasing factor in patients with TS Reference Chappell, Leckman, Goodman, Bissette, Pauls, Anderson, Riddle, Scahill, McDougle and Cohen[8]. This further suggests that there may be secondary sympathetic activation as well as elevated beta-endorphin release Reference Koob[9]. Alterations in other neurotransmitter, including cholinergic, gamma-aminobutyric acid (GABA)-ergic, and serotonergic, systems are also thought to play a role in the pathophysiology of TS Reference Leckman, Bloch, Smith, Larabi and Hampson[10].
Although pharmacological treatment can be effective for the symptoms of TS, use of medications, such as antipsychotics and alpha2-adrenergic agonists, can be limited by their side effects Reference Roessner, Plessen, Rothenberger, Ludolph, Rizzo, Skov, Strand, Stern, Termine, Hoekstra and ESSTS Guidelines Group[3]. In particular, antipsychotics are known for their cardiovascular, metabolic, and neuro-motor side effects Reference Roessner, Plessen, Rothenberger, Ludolph, Rizzo, Skov, Strand, Stern, Termine, Hoekstra and ESSTS Guidelines Group[3]. There have also been rare cases of antipsychotic-associated worsening of pre-existing tics or induction of new tics in individuals with tic disorders [Reference Nath, Bhattacharya, Hazarika, Roy and Praharaj11, Reference Diaz, Smith and Maccario12]. Therefore, alternative or adjunct nonpharmacological treatment can be of substantial value in people living with TS. For instance, behavioral therapy that involves tic-awareness (i.e., observing the premonitory urge or other signs preceding the occurrence of a tic) and competing-response (i.e., engaging in a voluntary behavior that is physically incompatible with the tic to manage the premonitory urge) training has already demonstrated efficacy in TS Reference Piacentini, Woods, Scahill, Wilhelm, Peterson, Chang, Ginsburg, Deckersbach, Dziura, Levi-Pearl and Walkup[13]. A recent meta-analysis of 8 randomized controlled trials (N = 438) revealed a medium-to-large treatment effect for behavioral therapy in persons with TS Reference McGuire, Piacentini, Brennan, Lewin, Murphy, Small and Storch[14].
However, little is known about physical activity (PA) or exercise. Although routine PA has demonstrated efficacy in a number of mental disorders Reference Zschucke, Gaudlitz and Exercise[15], limited evidence exists for TS. To our knowledge, there is no randomized controlled trial examining the effects of PA or exercise in TS. Questionnaires and self-reports have revealed that PA was helpful in attenuating tics in up to 32% of youth and adults with TS [Reference O'Connor, Brisebois, Brault, Robillard and Loiselle16, Reference Conelea and Woods17]. In other studies, however, PA as an attenuating factor was endorsed only in 7-9% of cases Reference Caurín, Serrano, Fernández-Alvarez, Campistol and Pérez-Dueñas[18]. Despite such a paucity of evidence, some health organizations have already been promoting PA for the management of tics [19–Reference Chansky and Niner22]. Although there is irrefutable evidence of health-related benefits associated with routine PA Reference Warburton, Nicol and Bredin[23], more evidence seems to be required for TS. We conducted a systematic review of the literature to better characterize the effects of PA or exercise, either acute or chronic, on the symptoms of TS.
2 Methods
2.1 Data sources and searches
This systematic review was conducted on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement Reference Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche, Ioannidis, Clarke, Devereaux, Kleijnen and Moher[24]. A comprehensive search of the literature was performed using the Medline, Embase, and PsycINFO databases to find all available publications until 12 August 2017. Reference lists from identified studies were also examined. Keywords and MeSH terms used were as follows: “physical activity” or “exercise” or “exercise therapy” or “physical exertion” or “sport*” and “tics” or “tic disorder*” or “Tourette*.”
2.2 Eligibility criteria
To be included in our systematic review, studies had to meet the following criteria: i) participants were diagnosed with tic disorders or TS, ii) participants were exposed to certain types of PA or exercise, iii) the outcome measure included the change in severity or frequency of tics in response to PA or exercise, and iv) randomized controlled trials, observational studies, and case reports were included. Studies were not excluded on the basis of age, sex, or ethnicity of participants. Studies were excluded if the type of PA or exercise protocol was not specified.
2.3 Data extraction
Descriptive data were extracted according to: i) author, year, country, study design, sample size, sex, age, diagnosis, and medication, ii) type of PA or exercise or training protocol, and iii) change in severity or frequency of tics in response to PA or exercise. If the intensity of PA or exercise was not specified by the study, we referred to the 2011 Compendium of Physical Activities to obtain a metabolic equivalent (MET) value for a certain type of PA and then the 2011 American College of Sports Medicine Position Stand to identify the absolute intensity of the given MET value [Reference Ainsworth, Haskell, Herrmann, Meckes, Bassett, Tudor-Locke, Greer, Vezina, Whitt-Glover and Leon25, Reference Garber, Blissmer, Deschenes, Franklin, Lamonte, Lee, Nieman and Swain26].
3 Results
Out of 343 studies screened, 8 studies were included in this review (Fig. 1). Of the 8 included studies, 5 studies reported the effects of acute PA [Reference Jacombe27–Reference Nixon, Glazebrook, Hollis and Jackson30, Reference Liu, Wang, Hsu, Wong, Chen and Lien33], and 4 studies reported the effects of chronic PA on tic symptomology [Reference Simms31–Reference Packer-Hopke and Motta34]. The studies are summarized in Table 1 and in detail in the following section. Overall, there was a trend that the effects of acute PA are intensity-dependent, where light intensity may alleviate and vigorous intensity may exacerbate tics. Chronic PA, however, appears to improve tics even at higher intensity. The relationship between PA intensity and tic expression is presented in Table 2. Lastly, there is some evidence that tics remain reduced after exercise but tends to be short-term.
3.1 Effects of acute physical activity
3.1.1 Light intensity
In the study by Nixon et al. Reference Nixon, Glazebrook, Hollis and Jackson[30], 18 young participants with TS (13 male, 5 female; age range: 10–20 years) completed an acute bout of aerobic exercise (i.e., aerobic kickboxing) at an easy followed by a more cognitively demanding (hard) level. Six of the 18 participants were being treated with one or a combination of 2–3 medications, including clonidine, aripiprazole, risperidone, and sertraline. Although the exercise session was designed to be moderately vigorous, participants overall performed at 43–63% of their estimated maximum heart rate (HRmax), which is estimated to be of light intensity according to the 2011 American College of Sports Medicine Position Stand Reference Garber, Blissmer, Deschenes, Franklin, Lamonte, Lee, Nieman and Swain[26]. Compared with baseline, there was a significant reduction in tic frequency during the exercise session (p = 0.001) as well as during the post-exercise session (p = 0.039). The authors noted that the reduction was greater during the easy than the hard level (p = 0.022); however, because the hard level was always followed by the easy level, the differential impact of the two tasks was difficult to establish Reference Nixon, Glazebrook, Hollis and Jackson[30]. The authors attributed the overall reduction in tic frequency to the activation of executive control circuits, sensory/motor tricks (i.e., gestes antagonistes), and/or reduced anxiety associated with exercise Reference Nixon, Glazebrook, Hollis and Jackson[30].
Garcia-Ruiz and del Val Reference Garcia-Ruiz and del Val[29] reported a case of a 76-year-old woman with chronic motor tics, who had been treated with tetrabenazine 12.5 mg tid. It was reported that a complete disappearance of her tics was noted when she engaged in sweeping floors. Sweeping may require intensities ranging from 2.3 METs (light intensity) to 3.8 METs (moderate intensity) for a 76-year-old woman [Reference Ainsworth, Haskell, Herrmann, Meckes, Bassett, Tudor-Locke, Greer, Vezina, Whitt-Glover and Leon25, Reference Garber, Blissmer, Deschenes, Franklin, Lamonte, Lee, Nieman and Swain26]. According to the supplementary video provided by the authors, it can be estimated that the woman was sweeping at light intensity. Other chores, such as ironing (1.8 METs) Reference Ainsworth, Haskell, Herrmann, Meckes, Bassett, Tudor-Locke, Greer, Vezina, Whitt-Glover and Leon[25], were also reported to partially attenuate her tics. The attenuating effect of sweeping on tic expression was attributed to its complex action that involves axial dorsal and cervical muscle groups, which may serve as a sensory/motor trick Reference Garcia-Ruiz and del Val[29].
Liu et al. Reference Liu, Wang, Hsu, Wong, Chen and Lien[33] reported a case of a 12-year-old boy with TS. The child complained of pain in his lower extremities, for which he went through a 3-month physical therapy program. It was reported that the child frequently engaged in the prescribed stretching exercises to inhibit his tics. Mild stretching generally requires 2.3 METs, which is equivalent to light intensity [Reference Ainsworth, Haskell, Herrmann, Meckes, Bassett, Tudor-Locke, Greer, Vezina, Whitt-Glover and Leon25, Reference Garber, Blissmer, Deschenes, Franklin, Lamonte, Lee, Nieman and Swain26]. The potential mechanism by which stretching promotes tic attenuation was not discussed in the study.

Fig. 1 Flow diagram of the included and excluded studies.
3.1.2 Moderate-to-vigorous intensity
Lombroso et al. Reference Lombroso, Mack, Scahill, King and Leckman[28] reported a case of a 17-year-old male with TS, who had been treated with clonidine 0.05 mg qid and fluoxetine 40 mg/day. In addition, the participant had obsessive-compulsive disorder and a history of heightened sensitivity to heat. Motor tic frequency (i.e., the number of tics per 2 minutes), body core (esophageal) temperature, and sweat rate were measured prior to, during, and after treadmill walking at 3.5 mph. There was a parallel increase in tic frequency, body core temperature, and sweat rate. Tics exacerbated to the extent that exercise was terminated prematurely after approximately 24 minutes. Walking at 3.5 mph generally requires 4.0 METs, which is equivalent to moderate intensity [Reference Ainsworth, Haskell, Herrmann, Meckes, Bassett, Tudor-Locke, Greer, Vezina, Whitt-Glover and Leon25, Reference Garber, Blissmer, Deschenes, Franklin, Lamonte, Lee, Nieman and Swain26]. The participant also reported that his tics often exacerbated when engaging in vigorous-intensity PA. Under identical conditions, the participant’s father, who had a diagnosis of TS but no history of heightened sensitivity to heat, did not exhibit any change in tic frequency during treadmill walking at 3.5 mph. The exacerbation of tics in the child was attributed to increased thermal stress associated with exercise Reference Lombroso, Mack, Scahill, King and Leckman[28].
Jacome Reference Jacombe[27] reported a case of a middle-aged man with mild TS. Whether the man was on medication was unknown. It was reported that his usual stereotyped, shouting utterances were exacerbated when he jogged medium distances despite feelings of well-being from exercise. Jogging generally requires 7.0 METs, which is equivalent to vigorous intensity [Reference Ainsworth, Haskell, Herrmann, Meckes, Bassett, Tudor-Locke, Greer, Vezina, Whitt-Glover and Leon25, Reference Garber, Blissmer, Deschenes, Franklin, Lamonte, Lee, Nieman and Swain26]. The exacerbation of tics associated with jogging was attributed to an exercise-induced release of endorphins (to be further discussed below) Reference Jacombe[27].
3.2 Effects of chronic physical activity
Wang et al. Reference Wang, Kuo and Stern[32] reported a case of an 11-year-old boy with TS. Whether the child was on medication was unknown. He engaged in a 6-month table-tennis training program that consisted of two 3-hour sessions per weekday. The intensity of training was not specified; however, it can be estimated that the overall intensity was moderate because table tennis generally requires 4.0 METs (i.e., moderate intensity) [Reference Ainsworth, Haskell, Herrmann, Meckes, Bassett, Tudor-Locke, Greer, Vezina, Whitt-Glover and Leon25, Reference Garber, Blissmer, Deschenes, Franklin, Lamonte, Lee, Nieman and Swain26]. The intensive design of the training program could have made it vigorous for the child, although the child never complained of being exhausted. The child’s Yale Global Tic Severity Scale (YGTSS) score changed from a moderate to minimal level (36–5). The improvement in the severity of the child’s tics was attributed to improved regulation of the cortico-striato-thalamo-cortical circuitry associated with repetitive practice of PA Reference Wang, Kuo and Stern[32].
In the aforementioned case report by Liu et al. Reference Liu, Wang, Hsu, Wong, Chen and Lien[33], the 12-year-old boy with TS, who complained of pain in his lower extremities, went through a 3-month physical therapy program that consisted of one 2-hour session per week of combined exercise modalities, including aerobic, resistance, and flexibility exercises. The intensity of training was not specified; however, it can be estimated that the intensity could have reached a vigorous level because each session consisted of running for 20 minutes in addition to other exercises for up to 2 hours. After the physical therapy program, the child’s pain in the lower extremities reduced and his YGTSS score changed from a moderate to mild level (37–28) “without taking anti-tic medications.” The improvement in the severity of the child’s tics was attributed to better interaction between the child and his mother and/or reduced pain associated with physical therapy Reference Liu, Wang, Hsu, Wong, Chen and Lien[33].
Table 1 Summary of studies examining tic response to acute or chronic physical activity.
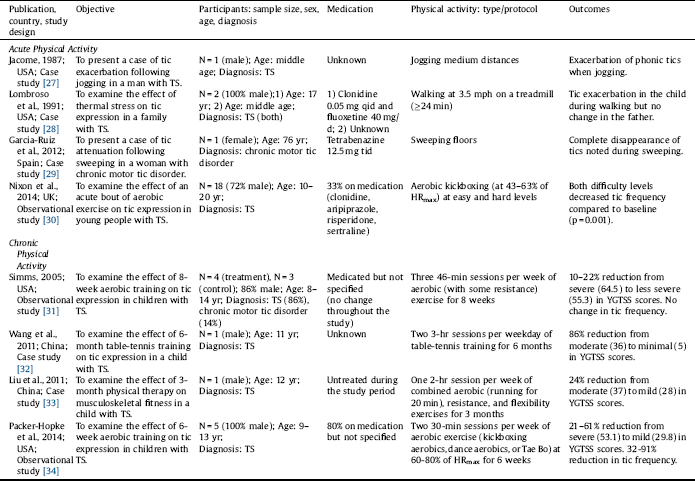
%HRmax: percentage of maximum heart rate; qid: four times a day; tid: three times a day; TS: Tourette syndrome; YGTSS: Yale Global Tic Severity Scale.
Table 2 Tic response to varying intensities of physical activity.
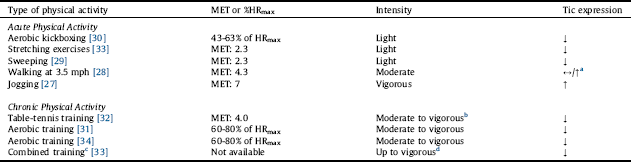
%HRmax: percentage of maximum heart rate; MET: metabolic equivalent; MET values were obtained from the 2011 Compendium of Physical Activities, and intensities were determined using the 2011 ACSM Position Stand.
a Note that the increase in tic expression refers to a boy who had a history of heightened sensitivity to heat.
b The vigorous-intensity part comes from the intensive design of the training program.
c Combined aerobic, resistance, and flexibility exercises.
d The vigorous-intensity part comes from the 20-min running component of the exercise session.
In the preliminary study by Simms Reference Simms[31], 7 participants with TS (age range: 8-14 years) were assigned to an 8-week aerobic exercise program (n = 4) or to a control group (n = 3). No changes in medications (not specified) were reported. The exercise program consisted of three 46-minute sessions per week of exercise modalities that included mostly aerobic and some resistance training at 60-80% of HRmax (moderate-to-vigorous intensity). Although the exercise program did not have a significant effect on tic frequency, there were noticeable decrements of approximately 10–22% in tic severity. The author suggested that exercise training may improve tics by increasing serotonin levels and cerebral blood flow, regulating the dopaminergic system, and reducing stress Reference Simms[31].
In the preliminary study by Packer-Hopke and Motta Reference Packer-Hopke and Motta[34], 5 male participants with TS and obsessive-compulsive disorder (age range: 9–13 years) engaged in a 6-week aerobic exercise program. Four of the 5 participants were on anti-tic medications (not specified). The exercise program consisted of two 30-minute sessions per week of aerobic exercise (i.e., aerobic kickboxing, dance aerobics, or Tae Bo) at moderate-to-vigorous intensity (i.e., 60–80% of HRmax). After the exercise program, there were reductions in YGTSS scores that ranged from 21 to 61% (i.e., from a severe to mild level) and in Tourette’s Disorder Scale-Parent Rated (TODS-PR) scores that ranged from 9 to 39%. Also, reductions in the number of tics per 5 minutes ranged from 32 to 91%. The improvement in tic expression was attributed to reduced anxiety associated with exercise Reference Packer-Hopke and Motta[34].
3.3 Lasting effects of physical activity
Three of the 8 included studies further examined whether exercise-associated alleviation of tics was sustained after the intervention [Reference Nixon, Glazebrook, Hollis and Jackson30, Reference Simms31, Reference Packer-Hopke and Motta34]. Nixon et al. Reference Nixon, Glazebrook, Hollis and Jackson[30] noted that tic frequency remained reduced for approximately 30 minutes following the bout of exercise. In the study by Simms Reference Simms[31], a 6-month follow-up survey revealed that the 8-week aerobic exercise program was associated with a sustained, but short-term, reduction in tic frequency in 75% of the participants. Packer-Hopke and Motta Reference Packer-Hopke and Motta[34] assessed their participants for additional 4 weeks after the 6-week aerobic exercise program. Although all participants’ YGTSS scores at follow-up were lower than their average baseline scores, tic severity and frequency returned to the baseline level for 2 participants (40%) based on the TODS-PR and for 3 participants (60%) based on the number of tics per 5 minutes.
4 Discussion
There is still a paucity of research on the current topic owing to the absence of randomized controlled trials. Nevertheless, several points can be drawn from this review: 1) PA at light intensity may acutely alleviate tics, 2) PA at vigorous intensity may acutely exacerbate tics, 3) chronic PA participation may improve tics even at higher intensity, and 4) tics can return to their baseline severity or frequency after discontinuation of chronic PA.
4.1 Acute physical activity
Different types of light-intensity PA (e.g., stretching exercises) or PA at light intensity (e.g., sweeping and aerobic exercise at 43–63% of HRmax) were reported to have positive effects on tics [Reference Garcia-Ruiz and del Val29, Reference Nixon, Glazebrook, Hollis and Jackson30, Reference Liu, Wang, Hsu, Wong, Chen and Lien33]. Suggested mechanisms include sensory/motor tricks and reduced anxiety and stress. PA that requires the use of certain muscle groups and body movements may act as a trick (i.e., gestes antagonistes) that simply replaces tics [Reference Wojcieszek and Lang35, Reference Gilbert36]. Acute bouts of exercise may offer additional benefits by positively influencing moods, which is relevant to TS as anxiety and stress are key factors that exacerbate tics Reference Conelea and Woods[17]. Nixon et al. Reference Nixon, Glazebrook, Hollis and Jackson[30] found that reductions in tic frequency corresponded with improvements in perceived anxiety and mood after an acute bout of aerobic exercise at light intensity. This is consistent with other studies demonstrating reduced levels of anxiety and increased levels of positive affect following acute bouts of exercise at light intensity [Reference Blacklock, Rhodes, Blanchard and Gaul37, Reference Bibeau, Moore, Mitchell, Vargas-Tonsing and Bartholomew38]. Vigorous-intensity PA, such as jogging, can also offer positive affect; however, according to the case report by Jacombe Reference Jacombe[27], feelings of well-being from vigorous exercise may be outweighed by other exercise-related factors that may exacerbate tics (discussed below).
Higher-intensity PA seems to have a different effect on tics. Understanding the pharmacology of anti-tic medications and exercise physiology may help explain the potential association of vigorous-intensity PA with tic exacerbation. Agents that agonize the presynaptic alpha2-adrenergic receptor, such as clonidine, are often prescribed for the treatment of TS Reference Roessner, Plessen, Rothenberger, Ludolph, Rizzo, Skov, Strand, Stern, Termine, Hoekstra and ESSTS Guidelines Group[3]. These agents inhibit norepinephrine release from the presynaptic terminal, suggesting that there may be heightened central noradrenergic activity in TS [Reference Hawksley, Cavanna and Nagai6–Reference Chappell, Leckman, Goodman, Bissette, Pauls, Anderson, Riddle, Scahill, McDougle and Cohen8]. Given the evidence that norepinephrine release is proportional to the intensity of PA [Reference McMurray, Forsythe, Mar and Hardy39–Reference Nagao, Suzui, Takeda, Yagita and Okumura42], vigorous-intensity PA may acutely exacerbate tics by stimulating central noradrenergic activity. Also, mu-opioid receptor antagonists, such as naloxone and naltrexone, have been shown to reduce tics [Reference Sandyk43–Reference Kurlan, Majumdar, Deeley, Mudholkar, Plumb and Como46], suggesting that endogenous endorphins may also play a role in the pathophysiology of TS [Reference Chappell, Leckman, Goodman, Bissette, Pauls, Anderson, Riddle, Scahill, McDougle and Cohen8, Reference Koob9]. Studies show that these peptides are released in a significant amount only after vigorous-intensity PA [Reference McMurray, Forsythe, Mar and Hardy39, Reference Nagao, Suzui, Takeda, Yagita and Okumura42, Reference Goldfarb and Jamurtas47], which may provide another explanation for tic exacerbation in response to vigorous-intensity PA Reference Jacombe[27]. Similarly, exercise (particularly at vigorous intensity) can significantly increase serotonin levels Reference Zimmer, Stritt, Bloch, Schmidt, Hübner, Binnebößel, Schenk and Oberste[48]. Although this may be of benefit to individuals with TS as serotonin levels tend to be reduced in this population [Reference Simms31, Reference Comings49], vigorous-intensity exercise can cause fatigue, for which an increased serotonin-to-dopamine ratio is believed to be a central mediating factor Reference Meeusen, Watson, Hasegawa, Roelands and Piacentini[50]. Fatigue is another factor highly associated with tic exacerbation Reference Conelea and Woods[17]. Vigorous-intensity PA can also cause thermal stress that may exacerbate tics Reference Lombroso, Mack, Scahill, King and Leckman[28].
4.2 Chronic physical activity
Although the effects of acute PA on tics may be intensity-dependent, studies that examined the effects of chronic PA (ranging from 6 weeks to 6 months) demonstrated improvements in tic symptomology even at moderate-to-vigorous intensity [Reference Simms31–Reference Packer-Hopke and Motta34]. This may be explained by several physiological adaptations associated with physical training. In TS, the evidence that alpha2-adrenergic receptor agonists can treat tics suggests that autonomic imbalance (i.e., heightened central noradrenergic activity) may be associated with the illness [Reference Roessner, Plessen, Rothenberger, Ludolph, Rizzo, Skov, Strand, Stern, Termine, Hoekstra and ESSTS Guidelines Group3, Reference Hawksley, Cavanna and Nagai6–Reference Chappell, Leckman, Goodman, Bissette, Pauls, Anderson, Riddle, Scahill, McDougle and Cohen8]. Thus, the observed improvements in tic expression may be explained by sympathoinhibition and enhanced vagal outflow associated with physical training Reference Mueller[51]. Also, dysregulation of the dopaminergic system may be another pathophysiological mechanism of TS. Studies using positron emission tomography suggest that amphetamine-induced striatal dopamine release is increased in individuals with TS [Reference Wong, Brasić, Singer, Schretlen, Kuwabara, Zhou, Nandi, Maris, Alexander, Ye, Rousset, Kumar, Szabo, Gjedde and Grace4, Reference Denys, de Vries, Cath, Figee, Vulink, Veltman, van der Doef, Boellaard, Westenberg, van Balkom, Lammertsma and van Berckel5]. There is evidence that physical training lowers basal extracellular dopamine levels as well as amphetamine-induced dopamine release in the rat striatum [Reference Meeusen, Smolders, Sarre, de Meirleir, Keizer, Serneels, Ebinger and Michotte52, Reference Marques, Vasconcelos, Rolo, Pereira, Silva, Macedo and Ribeiro53]. Moreover, a meta-analysis that included 2914 sedentary patients with a chronic illness has demonstrated that exercise training improves anxiety Reference Herring, O'Connor and Dishman[54], which is a key factor that exacerbates tics Reference Conelea and Woods[17].
Chronic or routine PA can be of benefit not only to the symptoms of TS, but also to the side effects associated with anti-tic medications. According to the 2011 European clinical guidelines for Tourette syndrome, antipsychotics, such as risperidone, and alpha2-adrenergic receptor agonists, such as clonidine, are highly recommended by experts for the treatment of tics in children and adolescents Reference Roessner, Plessen, Rothenberger, Ludolph, Rizzo, Skov, Strand, Stern, Termine, Hoekstra and ESSTS Guidelines Group[3]. However, these medications are frequently associated with cardiovascular adverse effects, in particular orthostatic hypotension Reference Roessner, Plessen, Rothenberger, Ludolph, Rizzo, Skov, Strand, Stern, Termine, Hoekstra and ESSTS Guidelines Group[3]. Exercise can counteract this side effect by increasing plasma volume (e.g., through aerobic training) and increasing muscle tone and thereby reducing venous pooling (e.g., through leg resistance training) [Reference Mtinangi and Hainsworth55, Reference van Lieshout56]. Also, antipsychotics that are frequently used to treat tics, such as risperidone and haloperidol, bind strongly to dopamine D2 receptors, increasing the risk of extrapyramidal symptoms. An animal study has shown that physical training can alleviate haloperidol-induced extrapyramidal-like symptoms in rats Reference Baptista, de Senna, Paim and Saur[57]. Lastly, exercise can effectively manage cardiometabolic side effects (e.g., weight gain and dyslipidemia), which occur commonly with the use of second-generation antipsychotics, such as clozapine, olanzapine, and risperidone [Reference Correll, Manu, Olshanskiy, Napolitano, Kane and Malhotra58, Reference Lang, Barr and Procyshyn59].
4.3 Limitations
There are limitations that require consideration. First, there is no randomized controlled trial to date examining the effects of PA on the symptoms of TS. Second, the majority of the included studies (i.e., 5 of the 8 studies) are case reports. The remaining 3 studies had small sample sizes and only one of them included a control group. Third, other than 3 studies, the relative intensity of PA was not provided via objective measures. However, this limitation could be addressed by approximating MET values using the 2011 Compendium of Physical Activities Reference Ainsworth, Haskell, Herrmann, Meckes, Bassett, Tudor-Locke, Greer, Vezina, Whitt-Glover and Leon[25].
4.4 Conclusion
This review points to a trend that the effects of acute PA are intensity-dependent in TS, where light-intensity PA may alleviate and vigorous-intensity PA may exacerbate tics. However, chronic PA appears to be beneficial even at higher intensity. It should be noted, however, that there is still a paucity of evidence on this topic due to the absence of randomized controlled trials. Nevertheless, with current evidence, it is recommendable that individuals with TS should first engage in PA at light intensity and gradually increase it. When tics become severe during vigorous-intensity PA, one should attempt to decrease the intensity and gradually increase it back. As tics can return to their baseline levels after discontinuation of PA, one should engage in PA on a routine basis. Doing so may not only reduce tics, but will also offer a myriad of other mental and physical health benefits [Reference Zschucke, Gaudlitz and Exercise15, Reference Warburton, Nicol and Bredin23]. Future randomized controlled trials should investigate the effects of different intensities and types of PA on tic symptomology. Studies should also better characterize other components of exercise prescription, such as exercise frequency and duration, in individuals with TS.






Comments
No Comments have been published for this article.