INTRODUCTION
Adenoviruses are medium-sized (90–100 nm), non-enveloped, double-stranded DNA viruses that can infect a broad range of vertebrate hosts worldwide. At least 57 distinct serotypes of adenoviruses and seven adenoviral species (A–G) have been detected in humans since they were first isolated in 1953 [Reference Rowe1, Reference Smith2]. Human adenoviruses (HAdVs) cause a wide range of illnesses, including ocular, respiratory and gastrointestinal infections, which vary in their severity, depending on the serotype [Reference Lenaerts3–Reference Lynch5]. HAdV subgroups B and D are mainly responsible for ocular infections, including epidemic keratoconjunctivitis (EKC), pharyngoconjunctival fever (PCF) and acute haemorrhagic conjunctivitis (AHC) [Reference Lynch5–Reference Ishiko and Aoki9]. The predominant serotypes differ among countries/regions and change over time. In recent years, the transmission of novel strains between countries or across continents, in addition to the replacement of dominant serotypes with new strains, has been reported. A series of adenovirus-related conjunctivitis outbreaks has been reported worldwide, with different epidemiological patterns.
Currently, there are no effective vaccines against ocular infections caused by adenoviruses [Reference Sundmacher10]. To date, few countries, with the exception of Japan and the United States, have established adenovirus surveillance systems [Reference Aoki and Tagawa11, Reference Nakamura12]. The present study aimed to compare the epidemiological features and viral evolution of EKC, AHC and PFC outbreaks based on 83 adenovirus hexon sequences and 76 outbreaks in the past 60 years. This research will help to improve our current understanding of conjunctivitis and to establish a suitable strategy for the control and prevention of HAdV-associated ocular infection.
METHODS
Outbreak data and hexon gene sources
Epidemiological data on 76 adenovirus-associated ocular outbreaks (48 EKC, 17 PCF and 11 AHC outbreaks) from 1953 to 2013 were obtained from PubMed Central (http://www.ncbi.nlm.nih.gov/pubmed/), as well as from the website of the WHO (http://www.who.int/en). Eighty-three representative HAdV hexon sequences collected from ocular and respiratory outbreaks were retrieved from GenBank (https:// www.ncbi.nlm.nih.gov/genbank/). Hexon sequences were selected using the following criteria: (1) sequences generated from low- and high-virulence/pathogenic strains; (2) sequences of representative strains responsible for outbreaks of ocular and respiratory infections; (3) sequences of different serotypes in different years; (4) sequences from high-circulating areas/countries and low-circulating areas/countries; (5) vaccine strains were contained in this study as the reference strains. The sequence accession numbers and genotypes are listed in Supplementary Table S1.
Homology and phylogenetic tree of the hexon gene
The sequences were aligned with Clustal X (ftp://ftp-igbmc.u-trasbg.fr/pub/ClustalX/) to determine their identity or similarity. Phylogenetic analyses of the aligned sequences were inferred based on neighbour-joining and maximum-likelihood methods using Mega 5.05 (http://www.megasoftware.net) and PHYLIP v. 3.67 software (http://evolution.gs.washington.edu/phylip.html). The plot similarity vs. position and mosaic sites was performed with SimPlot v. 3.5.1 [Reference Lole13] (the Johns Hopkins School of Medicine, USA) to determine the degree of identity of the query sequence (HAdV-7 vaccine strain) to a panel of reference sequences comprised of 8 hexon sequences from ocular infections in a sliding window (windows 200 bp/step). To deduce the common ancestor, a hexon sequence network was constructed using Network v. 4.611 software (Fluxus Technology Ltd, UK).
Structure modelling of hexon proteins
Three protein query Hexon sequences, an EKC infection strain (AB330133), one respiratory infection strain (JF800905) [Reference Tang14] and a respiratory infection HAdV-7 vaccine strain (AY594256) [Reference Purkayastha15], were uploaded to the SWISS-MODEL site (http://swissmodel.expasy.org/workspace/). The three-dimensional (3D) structure of hexon proteins was constructed based on the template 2OBE (1·45 Å) (ID). A hexon sequence of a representative ocular infection strain was selected from an EKC outbreak of a high-prevalence area. The predicted protein structure of the strains was superimposed on that of the retrieved protein templates using Visual Molecular Dynamics v. 1.8.6 software [Reference Wu16].
Statistical analysis
The locations of the PCF and AHC outbreaks were geocoded by the Google Map geocoding service (https://google-developers.appspot.com/maps/documentation/javascript/examples/geocoding-simple), and the x (longitude) and y (latitude) coordinates were recorded. Then, ArcGIS (ArcMap, v. 10.2, ESRI Inc., USA) was used to generate a spatial distribution map.
RESULTS
Hexon gene phylogeny and homology
The homology analyses based on the alignment of hexon sequences from the HAdV-associated ocular infections showed that the ocular strains were closely related (75·3–100% homology) compared to 41–50·3% similarity for those involved in respiratory infection. The phylogenetic analyses of the serotypes revealed an ocular HAdV cluster in the three subgenetic lineages that was very different from those obtained from the lung (Fig. 1). The plot similarity vs. position showed that the percentage of homology was 50–92% in position nucleotide (nt) 0–400, 50–66% in position nt 400–800, 50–84% in position nt 800–1200, 50–86% in position nt 1200–2400 and 66–90% in the positions after nt 2400 regions, respectively (Supplementary Fig. 1). In addition, nt 400–800 was highly diverse compared to that of the other regions. A median-joining network analysis showed that the 83 HAdV isolates from the respiratory and ocular infections had no common ancestor and that they could be segregated into three subgroups. No geographical/regional clusters were identified in any of the sublineages in any of the years (Fig. 2, Supplementary Table S2).

Fig. 1. Phylogenetic tree based on the hexon gene of HAdV strains isolated from ocular, respiratory and vaccine strains retrieved from GenBank, listed in Supplementary Table S1. Subgroup 1, blue; subgroup 2, yellow; subgroup 3, purple).

Fig. 2. Median-joining network depicting the relationships between the respiratory, ocular and vaccine strains between 1953 and 2013. The analysis is based on hexon gene sequences of 83 isolates deposited in GenBank from worldwide outbreaks between 1953 and 2013. The red points denote hereditary points. The yellow points denote hexon sequences. Information on the haplotype distribution is provided in Supplementary Table S2.
3D structure prediction of the hexon protein
To compare the structural modelling of the dominant neutralizing epitope functionally residing in the single capsid polypeptide (amino acids 103–111, 369–378, 589–600), representative isolates from the ocular (AB330133, Ad19), respiratory (F800905, Ad7) and vaccine (AY594254, Ad4) strains were analysed. The 3D structure showed that the major HAdV capsid protein (hexon) of the different strains all possessed a β-barrel structural motif, which included three variable loops, a silk-like random coil, helices, a folded sheet and a rolled angle. The same motif has been found in icosahedral capsids of diverse DNA viruses [Reference Smith2]. The dominant neutralizing epitope was located on the outer surface of the capsid in all three strains (a yellow loop indicates amino acids 103–111, a red loop indicates amino acids 369–378, and a green loop indicates amino acids 589–600). The configuration of the three loops in the respiratory strain and its vaccine strain was very similar, with the green and yellow loops inter-related together with the red loop appearing to be more separated. In contrast, the three loops were significantly separated in the hexon protein from the ocular infection strain, where the red loops were the most important epitope (Fig. 3).

Fig. 3. Prediction of the 3D structure of three protein query hexon sequences of (a), an ocular infection strain AB330133; (b), a respiratory infection strain F800905; and (c), a vaccine strain AY594254. The yellow sites indicate amino acids 103–111. The red sites denote amino acids 369–378. The green sites indicate amino acids 589–600.
Epidemiological features of the EKC, PCF and AHC outbreaks
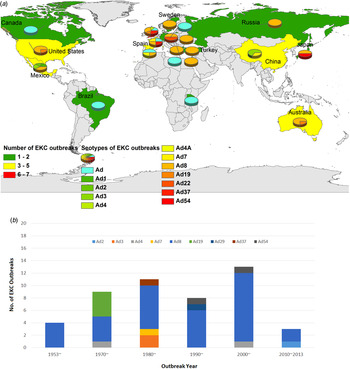
Forty-eight EKC outbreaks were reported worldwide, with most attributed to HAdV-8, -19, -37, -53 and -54 [Reference Frantzidou17–Reference Huang60]. As shown in Figure 4a , EKC outbreaks were widely reported in Europe, including the UK, France, Spain, Turkey, Greece, Sweden, Russia and Germany, with HAdV-8 being the most prominent strain. EKC outbreaks were also reported in Mexico, Brazil and Chile in Latin America, as well as in the United States and Canada in North America. EKC outbreaks were also reported in Africa and Asia, including India, China and Japan. Different HAdV serotypes were isolated from various outbreaks. In 1953–1969, serotype HAdV-8 accounted for 100% of all outbreaks. In 1970–1979, HAdV-8, -4 and -19 accounted for 44·44%, 11·11% and 4·44%, respectively, of all outbreaks. In 1980–1989, HAdV-8 accounted for 63·64% of outbreaks. HAdV-3, -37 and -7 were responsible for 18·8%, 9·09% and 9·09% of all outbreaks, respectively, in the same period. In 1990–1999, HAdV-8 was responsible for 77·27% of all outbreaks, and HAdV-29 and -37 were each responsible for 9·66% of all outbreaks. In 2000–2009, HAdV-8 accounted for 84·62% of all outbreaks, whereas HAdV-54 and -4 were each responsible for 7·69% of all outbreaks. Finally, in 2010–2013, HAdV-8 accounted for 66·67% of all outbreaks, and HAdV-2 accounted for 33·33% (Fig. 4b ). The occurrence of the EKC outbreaks fluctuated over time. Of the reported outbreaks between 1953 and 2013, 8·33% occurred before 1970. In the years 1970–1979, 1980–1989, 1990–1999, 2000–2009 and 2010–2013, the reported incidence was 18·75%, 22·92%, 16·67%, 27·08% and 6·25%, respectively. Public facilities, especially those related to the healthcare sector, played a pivotal role in the EKC outbreaks. Ophthalmology clinics were the most frequent sources of EKC and accounted for 65·22% of all outbreaks. Neonatal intensive care units (ICUs) accounted for 8·70% of infections, and infant daycare centres and multidisciplinary hospitals each accounted for 6·52%. Geriatric centres, community centres, industrial facilities and nursery schools also contributed to the EKC outbreaks (Fig. 5a ). Figure 5b shows the outbreaks were reported in different population sizes. In most of the EKC outbreaks, fewer than 100 people were infected. All age groups (4–85 years) were vulnerable to EKC infections during the outbreaks (data not shown).

Fig. 4. The geographical distribution (a) and timeline (b) of the 48 EKC adenovirus outbreaks from 1953 to 2013 worldwide. (Note: shadow of the geographical distribution map denotes the number of EKC outbreaks in each area or country. The colours green, yellow and red denote a low, medium and high number of EKC outbreaks, respectively. The pie charts show the percentage of the serotype composition in the EKC outbreak areas or country.)

Fig. 5. (a) Locations of the 48 HAdV-associated EKC outbreaks from 1953 to 2013. (b) The distribution of the 48 HAdV-associated EKC outbreaks from 1953 to 2013.
From 1953 to 2013, 17 large-scale PFC outbreaks were reported from different areas worldwide (Table 1). Most of these were caused by HAdV-3, -4, -7, -8 and -19, with HAdV-3 being the predominant strain. The positive rate ranged from 32·0% to 100% in the PFC outbreak samples. In 11 of 17 outbreaks, the outbreak usually infected a population size under 100. The distribution of the serotypes varied at different times and in different locations. With regard to the timing of the PFC outbreaks between 1953 and 2013, 5·88% were reported before 1970. The reported incidence in 1970–1979, 1980–1989, 1990–1999, 2000–2009 and 2010–2013 was 23·53%, 17·65%, 17·65%, 23·53% and 11·76%, respectively. Swimming pools were the most common source of the outbreaks. Summer camps were also implicated. PCF most commonly affected older teenagers.
Table 1. The virology and epidemiology of the global pharyngoconjunctival fever (PCF) outbreaks (N = 17) caused by different subtypes of human adenovirus from 1953 to 2013

n.a., Not available.
Since the first description of an AHC outbreak in Ghana in 1969 [Reference Chatterjee61], 11 outbreaks caused by the adenovirus have been reported. The main serotypes were HAdV-2, -4, -7, -8, -11 and -19. On average, 50 patients were affected in each outbreak. There was no predominant isolate and significant epidemical locations distribution. Interestingly, nine AHC outbreaks were associated with a concurrent infection, including picornavirus, EV70, coxsackievirus, herpes simplex virus (HSV) and chlamydia. The case-positive rate ranged from 8·33% to 53·33% in the AHC outbreak samples (Table 2). Similar to EKC, the AHC outbreaks were mostly linked to ophthalmology clinics, hospitals and crowded facilities, and the most vulnerable populations were those aged 15–40 years (Table 2).
Table 2. The virology and epidemiology of the global acute haemorrhagic conjunctivitis (AHC) outbreaks (N = 12) caused by different subtypes of human adenovirus from 1953 to 2013

CA24v, Coxsackie virus A24 variant; HSV, herpes simplex virus.
n.a., Not available.
DISCUSSION
Although previous studies have described adenovirus outbreaks associated with respiratory infections circulating throughout the world, a comprehensive analysis of ocular adenovirus infections has not been conducted. Ocular infections are largely restricted to type B and D adenoviruses (Supplementary Table S3). However, different serotypes of HAdV are associated with different disease manifestations, severity of infection, age and the underlying medical condition of the patients [Reference Lenaerts3]. The present study showed that ocular HAdV serotypes were highly divergent and polymorphic in distinct geographical areas and over time, with a predominance of HAdV-8, -19 and -37 strains worldwide [Reference D'Angelo62, 63].
Many ocular adenoviruses are endemic, and infections in patients in the absence of an outbreak are generally not reported due to the absence of surveillance systems in many countries. Thus, the current summary of the adenovirus-associated conjunctivitis outbreaks might be incomplete. In addition, most outbreaks are unreported because they are no longer novel, except when they are associated with a new type of adenovirus. The present study was initiated to describe the virology and epidemiology of reported conjunctivitis outbreaks caused by different adenovirus serotypes from 1953 to 2013, thereby extending and updating current knowledge on adenoviral-associated ocular infections. This study focused on the epidemic patterns and regularities of adenovirus associated-conjunctivitis circulating in different continents/countries, in addition to the seasonal distribution and populations affected and adenovirus subtypes.
The virological analysis supported findings in the current literature, showing that different serotypes of HAdV were associated with different types of conjunctivitis [Reference Yanoff and Jay64, Reference Adhikary65]. The adenovirus responsible for conjunctivitis was divided into three subgroups, with no common ancestral nodes in different countries over different years, as shown by the median-joining network analysis. This result suggests that the adenoviruses maintained their overall structural features, with minimum diversity with respect to locations and transmission mechanisms. As there are different serotypes of adenoviruses in conjunctival infections, repeated infection by different variants of the virus may occur.
The hexon of HAdV has several neutralizing epitopes. Of these, the anti-hexon antibody is the most effective in neutralizing HAdV infection. To compare the structural modelling of the dominant neutralizing epitope functionally residing in a single capsid polypeptide, we compared the hexon's sequences and 3D structure. The ocular-associated HAdV strains shared low identity of hexon sequences, in contrast to the strains isolated from respiratory infections and their vaccine strain, which shared high identity. Ocular HAdV strains shared lower identity of hexon sequences compared to strains isolated from respiratory infections and their vaccine strain. Similarly, the 3D structure of the antigen determinant site of the hexon protein varied in different isolates from the respiratory infection, a candidate for vaccine strain and ocular infection. The configuration of the three loops was very similar in the respiratory strain and its vaccine strain, where green and yellow loops inter-related together with the red loop appeared to be more separated. In contrast, the three loops were significantly separated in the hexon protein of the ocular infection strain. The red loop was the most important epitope, it might change the overall spatial structure in this ocular strain, which is different from the other two isolates. All these sites were related to the neutralizing antibody, which induced no cross-protection in the ocular infections, even after the administration of current available vaccines against the respiratory infections [Reference Madisch66].
There are different epidemiological features between various HAdV-associated conjunctivitis infections. EKC due to adenovirus infection is the most common form of keratoconjunctivitis, which was supported by the findings of the present study. In the present study, the EKC outbreaks were mainly associated with HAdV-8, -19, -37 and -54, with HAdV-8 being the predominant strain over time and geographical locations. The PCF outbreaks were associated with waterborne transmission of various adenovirus types, such as HAdV-3, -4 and -7, with HAdV-3 the most common aetiological agent. This was similar to the serotypes causing respiratory infections. The AHC outbreaks were mainly caused by HAdV-2, -7, -8 and -11, and they were usually associated with co-infection with other agents. Co-infection might improve their transmission capability and result in severe ocular symptoms and signs. It is still not clear whether HAdV infection was the first pathogen.
Outbreaks of conjunctivitis caused by adenoviruses have been reported worldwide [Reference Adhikary and Banik67]. EKC and AHC are the primary forms of conjunctivitis, especially in Asia. PCF outbreaks have often been reported in tropical and subtropical areas of Australia, as well as in the United States and China. In the present study, the geographical distribution of the outbreaks was related not only to the population density and the immune status of those affected but also to the local climate, level of available healthcare and economic status. The EKC and AHC outbreaks were most often linked to ophthalmology clinics and neonatal ICU wards, whereas the sources of the PCF outbreaks were inadequately chlorinated swimming pools and small lakes in summer seasons. With regards to the chronology the outbreaks, the EKC and PCF outbreaks periodically circulated in the study populations because of the accumulation of susceptible individuals and shifts in the serotypes of the adenovirus. In contrast, many AHC outbreaks were reported in 1970–1980, but the number of outbreaks then markedly decreased. Improvements in hygiene measures and early diagnoses may explain the decrease in the AHC outbreaks. The current study, which was based on a series of large-scale outbreaks, demonstrated that EKC was not associated with age, sex, ethnic origin, social status or nutritional status [Reference Meyer-Rusenberg68]. However, PCF infection was associated with young age (<18 years), and AHC was common in those aged 15–40 years. The different age distribution might be due to different serotypes varying in their affinity to the ocular epithelial cells, and different age groups have various protective antibody levels and also vary in their exposure risk. There are also difference in the outbreak size of EKC, PCF and AHC. Of the reported outbreaks, the AHC outbreaks were the largest, pointing to the highly contagious nature of the adenovirus responsible for this type of conjunctivitis.
Although an adenovirus vaccine has been administered to US military personnel since 1971, this vaccine only elicits immunity to serotypes 4 and 7. This concentred vaccine was evidenced by a reduction in the rate of febrile respiratory illness since its administration to recruits [Reference Croyle69]. As there is currently no specific chemotherapeutic agent or broadly effective adenovirus vaccine to protect against ocular infections, strict attention to infection-control practices is likely the most effective way to prevent outbreaks of adenovirus-associated diseases, such as EKC. Additionally, maintaining adequate levels of chlorination is necessary for preventing swimming pool-associated outbreaks of adenovirus conjunctivitis. As with many other illnesses, general hygiene measures, such as hand washing, are the best way to inhibit person-to-person transmission. Improvements in surveillance systems, diagnostic and therapeutic capacities and data handling and transfer are determining factors in the control and prevention of adenovirus-induced ocular infections. Delays in the identification and reporting of HAdV-associated conjunctivitis outbreaks and in the institution of infection-control measures can impede timely investigation and prolong transmission. It is important to establish a global adenovirus surveillance system, which should include virological and epidemiological surveillance. Such a system will aid the monitoring of adenovirus outbreaks and the detection of other unexpected novel adenoviruses that may emerge. The present study can improve our understanding of conjunctivitis and play a role in establishing a suitable strategy for the control and prevention of HAdV-associated ocular infections.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268815003246.
ACKNOWLEDGEMENTS
We are grateful to Professor Glen R. Nemerow and Professor Theresa McCarthy, Department of Immunology and Microbial Science, the Scripps Research Institute, USA for their invaluable advice in revising the manuscript.
This work was supported by the Fundamental Research Funds for the Central Universities (2015FZA7008), Technology Plan project of Zhejiang province (2012KYB090).
DECLARATION OF INTEREST
None.