INTRODUCTION
Pertussis (commonly known as whooping cough) is a vaccine-preventable bacterial respiratory infection caused by Bordetella pertussis which can be fatal in infants [Reference Warfel and Edwards1]. For older children and adults, symptoms are milder and often unrecognized as pertussis, but nonetheless associated with a considerable burden of illness [Reference van Hoek2]. About 25% of infections in previously fully susceptible individuals will be asymptomatic and have been considered to be without an onwards risk of transmission [Reference Schellekens, von König and Gardner3].
The introduction of whole-cell pertussis (wP) vaccination in 1957 led to a rapid reduction in pertussis incidence in England and Wales [Reference Campbell4]. However, after vaccine safety concerns in the 1970s and a marked decline in vaccine coverage, there followed a period of increased incidence that lasted until 1990, when confidence in vaccination was restored; vaccine coverage in England and Wales recovered to 92% by 1992 and has been sustained at that level or higher ever since [Reference Campbell4].
In 2001, acellular pertussis (aP) vaccine was introduced into the routine childhood immunization programme as a preschool booster dose in England and Wales, and in 2004 aP vaccine replaced wP vaccine in the primary schedule [Reference Campbell4]. There are different aP and wP vaccines available, of which certain formulations of each type appear equally efficacious against disease, although the aP vaccine is less reactogenic [Reference Miller5] but associated with more rapidly waning immunity [Reference Warfel and Edwards1]. After immune priming with multiple doses of aP vaccines, high levels of protection against disease are present for 4–12 years in children [Reference Wendelboe6], after which the risk of pertussis significantly increases [Reference Tartof7]. Consequently, the use of aP vaccines has been associated with a concomitant increase in pertussis in vaccinated adolescents [Reference Clark8,Reference Skoff and Martin9] and a general resurgence in disease in some countries [10]. In addition to waning immunity, a recent study using a baboon model of pertussis suggests that whereas wP-vaccinated individuals are protected against colonization, aP-vaccinated individuals, due to a qualitatively different immune response, are protected against disease but not against asymptomatic or mild infection with the potential for onward transmission [Reference Warfel, Zimmerman and Merkel11].
High coverage with pertussis-containing vaccines resulted in a prolonged period of effective disease control in England. However, in late 2011 a national outbreak of pertussis began which resulted in the largest increase in cases seen in over two decades, peaking in 2012. Following increased infant disease and deaths during this outbreak, a UK pertussis vaccination programme for pregnant women was introduced from October 2012. This was later shown to have a high effectiveness for infants born to vaccinated mothers [Reference Amirthalingam12,Reference Dabrera13]. Prior to the 2011 outbreak, a trend for increasing cases of pertussis in those aged ⩾15 years in England had been observed [Reference Campbell4], consistent both with increased case ascertainment in England [Reference Campbell4] and a change in global epidemiology [Reference Tan, Trindade and Skowronski14]. As the outbreak in England developed, a further marked increase in incidence rates in those aged ⩾15 years was observed [15].
In contrast to infants, where household contacts are the source for most cases [Reference Wiley16], for adults, the main sources of pertussis are considered to be their children and work colleagues [Reference De17]. Outbreaks of pertussis in healthcare settings have involved a number of different transmission routes, including transmission of pertussis between healthcare workers (HCWs) [Reference Pascual18], infection of HCWs through contact with patients with pertussis [Reference Baugh and McCarthy19], and a combination of both [Reference Bassinet20]. Studies have suggested that 1–6% of paediatric HCWs develop asymptomatic infection with B. pertussis [Reference Wright, Decker and Edwards21, Reference Cunegundes22], although seroprevalence may not differ from that of the general population [Reference Riffelmann23]. During the 2011 outbreak, booster vaccination of HCWs was considered as a control measure in order to reduce transmission of pertussis to neonates and young infants [24], but, as it is not considered the optimal strategy for reducing the burden of infection for infants, was not implemented. Evidence from France and Australia also suggests that vaccine uptake by HCWs can be low [Reference Bechini25, Reference Hope26].
In early 2012, as part of the public health response to the outbreak in England, a national prospective case-control study of laboratory-confirmed pertussis in persons aged ⩾15 years was undertaken. The aim of this study was to investigate whether employment within different sectors, professional contact with children and young adults, and household contact with children and young adults were independent risk factors for pertussis for those aged ⩾15 years.
METHODS
Study design
A case-control study of persons aged ⩾15 years in England registered with a general practice (GP). Target recruitment was 250 cases and 500 matched controls (1:2 ratio of cases to controls) to provide 80% power (at 5% significance) to detect a minimum odds ratio (OR) of 2·4 with a 5% prevalence of exposure in controls. Formal ethical approval was not required as the study was undertaken as part of the public health response to an outbreak.
Case definition
A case was defined as a person resident in one of four regions of England (Yorkshire and the Humber, South West, West Midlands, London) aged ⩾15 years with clinical signs or symptoms consistent with pertussis and either (1) a nasopharyngeal aspirate or per-nasal swab positive for B. pertussis by culture, (2) an anti-pertussis toxin IgG titre >70 IU/ml, or (3) a clinical specimen testing positive for B. pertussis by polymerase chain reaction. Cases with positive serology in the absence of isolation or detection of B. pertussis were excluded if they were known to have received vaccination for pertussis within the last year.
Study participant recruitment
Cases were recruited by local health protection teams within the four regions. Controls were recruited for each case according to GP, age group and sex. The GP of each case was asked to contact by post 10–15 suitable controls (each fifth name from the practice register of the same sex as the case and within the same 5-year age group). Each participating practice was provided with a set of pre-paid envelopes containing letters of invitation to participate which they were requested to send to the nominated controls. The GP was asked to exclude controls if the individual was assessed as inappropriate for inclusion or known to have previously had pertussis. If a GP declined to participate a neighbouring practice was selected and approached to nominate controls.
Data collection
Data were captured using an online questionnaire in SelectSurvey (https://selectsurvey.net). The questionnaire captured demographics, history of recent foreign travel (3 months prior to onset of illness for cases and interview date for controls), and selected questions designed to test three specific hypotheses related to the odds of having pertussis for individuals aged ⩾15 years: (1) employment within different sectors, (2) professional contact with children and young adults, and (3) household contact with children and young adults. Cases were interviewed by local health protection staff while controls were either interviewed by a member of the study team or self-completed using the online questionnaire. Each case plus associated controls was interviewed by the same interviewer wherever possible. All interviews were administered by local health protection and field epidemiology teams.
Statistical analysis
Univariable associations with outcome were calculated as unadjusted odds ratios (uOR). To explore confounding between two explanatory variables, Mantel–Haenszel odds ratios (ORMH) were calculated. Logistic regression was used to estimate adjusted odds ratios (aOR) for associations between explanatory variables and outcomes. Base variables of demographics (age quintiles, sex, region of residence) and recent foreign travel were included in all multivariable models irrespective of statistical significance. Five multivariable models were developed:
-
• M1: base variables only.
-
• M2: base variables + employment type variables.
-
• M3: base variables + professional contact with children/young adult variables.
-
• M4: base variables + household contact variables.
-
• M5: base variables + variables from all other categories.
For M1, two separate models were developed, specifying age either as a linear variable or using fractional polynomial transformations. The fit of these models was compared to the model where age was specified as quintiles using a likelihood ratio test (LRT). The most appropriate specification for age was then used for all subsequent models. For M2–4, a parsimony approach was taken for building multivariable models for each set of hypothesis testing variables. Firstly, a sub-model including all variables together (M2A, M3A, M4A) was constructed for each hypothesis in order to assess confounding and/or collinearity within each of the three variable sets. In order to determine the order variables were to be added for building the final parsimonious model for each hypothesis, variables were first added individually to the base variables (one model per variable; model set M2B, M3B, M4B). For construction of the final model for each hypothesis (M2C, M3C, M4C), variables were added to the base variables one at a time in order of increasing statistical significance based on the P value from model set B. Variables were retained in the final model for each hypothesis if their inclusion significantly improved fit (LRT, P < 0·05). For M5, variables from all three hypotheses with a univariable association with outcome of P < 0·2 were considered for inclusion in the same model. All of these variables were added simultaneously to the base variables and then removed in order of descending P value. The removal of each variable was assessed through a LRT comparing the reduced model to the previous one. The final model was reached when all variables additional to the base variables had an associated P < 0·05. The fit of final models for M1–5 was assessed using the Hosmer–Lemeshow goodness-of-fit test [Reference Hosmer and Lemeshow27]. All analysis was carried out in Stata v. 13.1 (StataCorp., USA).
RESULTS
Study participants
Study participants were recruited between 1 June and 31 October 2012. A total of 231 cases and 190 controls completed questionnaires and were eligible for inclusion in the study. As was expected for a case-control study of this nature, the response rate for potential controls was low (6·8% in the one study region where data on response rates was available). For the one study region where data was available on participation from a subset of GPs contacted, 1/42 (2·4%) of those practices declined to take part in control selection. The actual statistical power of the study was 80% to detect a minimum OR of 3·0 for 5% prevalence of exposure in controls. Descriptive characteristics of cases and controls are given in Table 1. Cases were on average slightly younger than controls (cases: mean age 43·5, range 15–87 years; controls: mean age 50·1, range 15–85 years). Data was missing only for three controls for region of residence.
Table 1. Characteristics of study participants: a case-control study of risk factors for pertussis in adults and teenagers in England
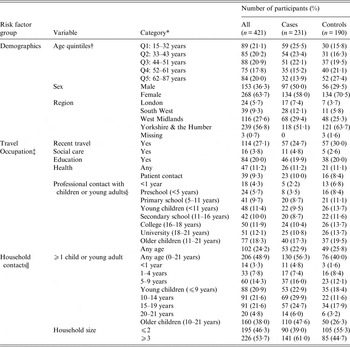
* Categories of missing data are only shown were ⩾1 case had missing data.
† Q1–Q5, quintiles 1–5.
‡ Studying or working in each specific area.
§ Working with children from specific age groups.
|| Individuals living within the same household.
Statistical analysis
Due to low levels of control recruitment, data were analysed using unconditional logistic regression with variables used for matching (age and sex) included in all models. Multivariable models were built in order to specifically test each of the three study hypotheses (Tables 2–4). Neither a linear specification of age nor a fractional polynomial transformation provided a significantly improved fit compared with age quintiles (both P > 0·05).
Table 2. Crude and adjusted associations between pertussis and occupational risk factors in adults and teenagers in England

uOR, Unadjusted odds ratio; CI, confidence interval; aOR, adjusted odds ratio; Q1–Q5, quintiles 1–5; Ref., reference group.
Values in bold indicate statistical significance (P < 0·05).
* All occupation variables added simultaneously to M1.
† A separate model produced for each occupation variable added to M1.
‡ Variables added to M1 in order of increasing statistical significance according to P value from M2B, variables retained only if the lead to a statistically significant improvement in fit.
§ χ 2 and associated P value of the likelihood ratio test of improved fit compared to M1.
Table 3. Crude and adjusted associations between pertussis and professional contact with children and young adults in adults and teenagers in England

uOR, Unadjusted odds ratio; CI, confidence interval; aOR, adjusted odds ratio; Q1–Q5, quintiles 1–5; Ref., reference group.
Values in bold indicate statistical significance (P < 0·05).
* All professional contact with children or young adult variables added simultaneously to M1.
† A separate model produced for each professional contact with children or young adult variable added to M1.
‡ Variables added to M1 in order of increasing statistical significance according to P value from M3B, variables retained only if the lead to a statistically significant improvement in fit.
§ χ 2 and associated P value of the likelihood ratio test of improved fit compared to M1.
Table 4. Crude and adjusted associations between pertussis and household contact in adults and teenagers in England

uOR, Unadjusted odds ratio; CI, confidence interval; aOR, adjusted odds ratio; Q1–Q5, quintiles 1–5; Ref., reference group.
Values in bold indicate statistical significance (P < 0·05).
* All professional contact with children or young adult variables added simultaneously to M1.
† A separate model produced for each professional contact with children or young adult variable added to M1.
‡ Variables added to M1 in order of increasing statistical significance according to P value from M3B, variables retained only if the lead to a statistically significant improvement in fit.
§ χ 2 and associated P value of the likelihood ratio test of improved fit compared to M1.
Associations between pertussis and employment within different sectors
None of the four variables considered (employment in social care, employment in education, employment in the health sector, employment with direct patient contact) were significantly associated with pertussis either in a univariable analysis or after adjustment for other variables in a multivariable model (Table 2). The final model (M2C) provided reasonable fit to the data [χ 2(82, n = 418) = 94·08, P = 0·171].
Associations between pertussis and professional contact with young children and adults
Two professional contact variables (contact with children aged <1 year, contact with preschool children) were significantly associated with outcome in a univariable analysis (Table 3). After adjustment within a multivariable model, only professional contact with children aged <1 year remained significant, with exposure associated with a reduced odds of pertussis [aOR 0·25, 95% confidence interval (CI) 0·08–0·78, P = 0·017]. The final model (M3C) provided reasonable fit to the data [χ 2(69, n = 418) = 77·39, P = 0·229].
Study participants were provided with the option of selecting more than one age group of children they worked with, and of the 24 participants who indicated they worked with preschool children, 14 (66·7%) also indicated they worked with children aged <1 year. The significant univariable association between working with preschool children is explained by confounding with also working with children aged <1 year: after stratification by working with children aged <1 year there was no significant association between pertussis and working with preschool children (ORMH 0·68, 95% CI 0·19–2·40, P = 0·370).
Associations between pertussis and household contacts with children and young adults
Three household contact variables (household contact with ⩾1 child or young adult aged 0–21 years, household contact with ⩾1 child aged 10–14 years, household contact with ⩾1 child or adult aged 10–21 years) and a total household size of ⩾3 persons were significantly associated with outcome during a univariable analysis (Table 4). Given the overlap between age groups, substantial collinearity in prediction was observed. After considering each variable in a stepwise forward selection approach, only household contact with ⩾1 child aged 10–14 years was included in the final model, with exposure associated with significantly increased odds of pertussis (aOR 2·61, 95% CI 1·47–4·64, P = 0·001). The final model (M4C) provided reasonable fit to the data [χ 2(89, n = 418) = 98·19, P = 0·237].
Total household size was no longer significantly associated with pertussis after adjusting for other household variables (model M4A), and was not included in the final model. The significant univariable association for total household size can be explained by confounding with household contact with ⩾1 child aged 10–14 years: after stratification by household contact with ⩾1 child aged 10–14 years there was no significant association between pertussis and total household size (ORMH 1·41, 95% CI 0·91–2·16, P = 0·124).
Adjusted associations between pertussis and all three categories of explanatory variables
The final multivariable logistic regression model, considering variables from all three categories of potential predictors, contained both variables significantly associated with outcome in final models from the category-based analysis: professional contact with children aged <1 year (aOR 0·24, 95% CI 0·07–0·76, P = 0·015) and household contact with ⩾1 child aged 10–14 years (aOR 2·66, 95% CI 1·48–4·79, P = 0·001) (Table 5). The final model (M6) provided reasonable fit to the data [χ 2(99, n = 418) = 108·49, P = 0·242]. The point estimates of adjusted associations differed by only 4·0% (professional contact with children aged <1 year) and 1·9% (household contact with ⩾1 child aged 10–14 years) from those of the category-only models.
Table 5. Crude and adjusted associations between pertussis and occupational, professional contact with children or young adults, and household contact risk factors for adults and teenagers in England

uOR, Unadjusted odds ratio; CI, confidence interval; aOR, adjusted odds ratio; Q1–Q5, quintiles 1–5; Ref., reference group.
Values in bold indicate statistical significance (P < 0·05).
* Variables added to M1 in order of increasing statistical significance according to P value of univariable association, variables retained only if inclusion led to a statistically significant improvement in fit.
DISCUSSION
This case-control study found two factors to be significantly associated with pertussis infection in adults and older teenagers in England: professional contact with children aged <1 year associated with reduced odds of pertussis, and sharing a household with ⩾1 child aged 10–14 years associated with increased odds of pertussis. Both of these factors were significant independent factors when contained within the same multivariable model, although both have a low prevalence in controls (6·8% reported professional contact with children aged <1 year, 11·6% reported living with ⩾1 child aged 10–14 years) and will represent relatively small population attributable fractions. In addition to these factors, we observed a significantly higher odds of pertussis in the South West region of England, reflecting the high number of cases in this area at the time of the study [15].
Due to the higher than expected number of cases without a corresponding control, and to maximise statistical power, data were analysed using unconditional logistic regression with variables used for selection of controls (age and sex) included in all multivariable models. Any bias associated with the use of an unmatched analysis for a design including matching is towards a null effect, returning conservative estimates of risk [Reference Breslow28]. As serological testing of controls was not undertaken, unrecognized or asymptomatic pertussis infection of controls cannot be excluded; it is certainly possible that individuals working with <1-year-olds may have developed mild or subclinical pertussis infection without having been aware. Although we did not collect pertussis vaccination history, and as such were unable to adjust for any potential confounding due to vaccination status, we are not aware of any obvious mechanism by which any confounding could occur; certainly, no programme has existed in England to offer pertussis booster vaccination to specific occupational groups or indeed to adults other than pregnant women (and only then offered from October 2012). There is therefore no obvious route through which pertussis vaccination could have been associated with either of the two factors found to be significantly associated with infection in this study.
Although HCWs have been implicated in transmission of pertussis within healthcare settings, we observed no significant association between working in a healthcare setting or direct patient contact with the odds of pertussis. We also found no similar association between working in education and social care. However, for all three exposures we cannot rule out misclassification of controls due to asymptomatic or mild pertussis infection. The lack of an association between working in healthcare and pertussis suggests that exposures for HCWs in our study do not differ significantly from that of the general population. Although a study in Brazil found that the seroprevalence of immunity to pertussis was higher in groups of HCWs working with children [Reference Cunegundes22], a study in Germany supports the findings of our study [Reference Riffelmann23].
While we did not observe a significant association between pertussis and working within a healthcare setting, we did observe a significantly reduced odds of pertussis for those individuals reporting professional contact with children aged <1 year. This association likely reflects frequent exposure to low levels of antigen that boost immunity to B. pertussis without causing a symptomatic infection [Reference Lavine, King and Bjornstad29]. Given that children aged <1 year consistently represent the age group with the highest incidence, including at the start of the 2011 pertussis increase in England [15], it is feasible that those working with children aged <1 year were more likely to have been immune-boosted by this mechanism prior to the increase in pertussis cases as the outbreak developed. No such association was observed for household contact with children aged <1 year, likely reflecting the relatively smaller number of individual children of this age group present within households compared with relevant professional settings.
There is good existing evidence to support the role of household members as the source of infant pertussis [Reference Bisgard30–Reference de Greeff32]. This study provides additional evidence that contact with children aged 10–14 years in the home is a significant risk factor for pertussis in older children and adults. For cases of adult pertussis, it is known that their own children and work colleagues are the most likely sources of infection [Reference De17]. Our findings suggest that it is specifically children aged 10–14 years, where susceptibility may have developed through waning of immunity following childhood vaccination [Reference Tartof7], that provide the greatest risk of household transmission of pertussis to older children and adults. Older children within the 10–14 years age group in this study may represent birth cohorts who received an aP booster vaccine and may be associated with more rapidly waning immunity compared with older birth cohorts vaccinated only with wP vaccines [Reference Sheridan33]. Certainly, at the start of the 2011 outbreak of pertussis in England there were higher than expected numbers of cases in teenagers and adults [34]. This study lacks the statistical power to investigate whether the risk of exposure to household contacts of specific ages within the 10–14 years age group is consistent.
Although there is empirical and modelling evidence that booster vaccination of adolescents is effective for reducing the incidence of pertussis in adolescents [Reference Lavine35], there is limited evidence of an indirect reduction of disease for infants [10], and no evidence of an indirect effect for older children and adults. Herd immunity following pertussis vaccination appears to be restricted to vaccinated cohorts only [Reference Lavine35]. A study in Australia found evidence of indirect effects in young, unimmunized infants only when adolescent booster vaccination formed part of a broader catch-up of all students within a high-school setting [Reference Quinn and McIntyre36]. However, assessing the impact of vaccination on the burden of illness for pertussis in older children and adults is problematic due to low case ascertainment rates and non-specific symptomatology.
The primary focus of the childhood pertussis vaccination programme is to prevent infant disease and evidence is emerging that adolescent vaccination does not provide indirect protection to infants. However, the results of this study highlight the importance of children aged 10–14 years in pertussis transmission to older adolescents and adults and support the need for further work to consider the potential public health benefit of reducing numbers of adult pertussis cases through the indirect effects of an adolescent booster vaccination. This may be particularly relevant now that the maternal vaccination programme has been shown to be highly effective at protecting young infants against disease.
ACKNOWLEDGEMENTS
We thank all study participants and the health protection and field epidemiology staff who contributed to this study. We also thank George Kafatos for commenting on the statistical analysis plan and the study steering committee for their contribution to planning.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.








