INTRODUCTION
There are more than 100 types of human papillomavirus (HPV) divided into three broad categories (high-risk types, probable high-risk types, low-risk types) depending on their oncogenic potential [Reference Munoz1]. Persistent infection with high-risk HPV and the integration of viral genomes into the host genome have been implicated in the aetiology of malignant and pre-malignant diseases of the female lower genital tract [Reference Remmink2]. A series of investigations found that specific types of HPV were closely associated with many kinds of cancers, such as laryngeal cancer, anal cancer, vulva cancer and bladder cancer [Reference Li3, Reference Hobbs4].
Many factors could increase the risk of HPV infection, for example, sexual behaviour, the number of both recent and lifetime male sexual partners, co-infection with other sexually transmitted diseases and vaginal infection, and early onset of sexual activity. Diet, smoking, genetics and immune suppression are also related to HPV infection [Reference Erickson5–Reference Vaccarella8].
It has been shown that pregnancy is a state of mild immunosuppression due to the decrease in natural killer cells or reduction in the helper T-cell type 1 cell-mediated response [Reference Weinberg9]. Sillman & Sedlis found that immunosuppressed women had a higher incidence of cervical neoplasia [Reference Sillman and Sedlis10]. Furthermore, Gloss et al. reported that the transcriptional promoter of HPV-16 contained a steroid hormone receptor-binding element that promoted HPV transcription, suggesting a hormonal activation effect on HPV replication [Reference Gloss11]. These observations indicated that the possible temporary altered state of immunity and the increased levels of steroid hormones during pregnancy might have an effect on HPV replication and its subsequent progression to disease development.
In addition, Merckx et al. [Reference Merckx12] reported that there was a significantly higher risk of children born to HPV-positive mothers becoming HPV positive, resulting in infantile anal and genital condyloma acuminatum, and juvenile laryngeal papillomatosis, although some HPV infections were almost cleared by age 6 months. Hence, the question regarding whether pregnant women are more susceptible to HPV infection than non-pregnant women is crucial. We hypothesize that pregnant women, with a state of mild immunosuppression [Reference Weinberg9], have a higher susceptibility to HPV infection, increasing the HPV prevalence in this population.
Until now, many observational studies have reported the risk of HPV infection in pregnant women, but the results are controversial. A few studies reported that pregnant women had a higher HPV prevalence [Reference Hernandez-Giron6, Reference Sillman13–Reference Aydin18]. However, several reports suggested a lower prevalence in pregnant women or no statistical difference compared to those of age-matched non-pregnant controls [Reference Soares19–Reference Schmeink26]. In addition, we did not find any articles estimating the age, trimester and type-specific prevalence of cervical HPV DNA in pregnant women. Therefore, we set out to estimate the prevalence of cervical HPV in pregnant women and clarify the risk of HPV infection in pregnant women by conducting a meta-analysis after a systematic literature review.
METHODS
Literature search
Articles published in English and Chinese were all considered. Articles in English were identified through PubMed, Medline, Elsevier Science, and Web of Science (ISI) databases from their earliest available date to 30 April 2013. The key words (‘human papillomavirus’ or ‘HPV’) and (‘pregnant’ or ‘pregnancy’ or ‘conceive’ or ‘conception’ or ‘gravidity’ or ‘be with child’ or ‘fetation’ or ‘to bear children’) were used in combination in order to retrieve the relevant literature in these databases. Articles in Chinese were identified through the China National Knowledge Infrastructure (CNKI), Database of Chinese Scientific and Technical Periodicals (VIP), Wan Fang database, and the China Biology Medical Literature database (CBM), four commonly used databases, which were searched from 1979, 1989, 1990, 1970 to 30 April 2013, respectively. Moreover, we reviewed the reference lists from retrieved articles to search for further relevant studies.
Inclusion criteria
Inclusion criteria were as follows: (1) the HPV prevalence in asymptomatic pregnant and non-pregnant women could be directly extracted or calculated from the original article. Pregnant women were those who attended a routine antenatal visit and non-pregnant woman were from a cervical cancer screening centre located at the same hospital; (2) if studies contained non-pregnant women, they were age-matched with pregnant women; (3) specimens for HPV detection were exfoliated cells that had been scraped or lavaged from the cervix and vagina; (4) information about HPV DNA types and detection methods had to have been clearly stated. If there were duplicate reports of the study, the article published earlier or providing the more detailed information was included.
Data extraction
The following information was extracted from each study: first author, journal and year of publication, country where the study was performed, study design, study period, the number of pregnant and/or non-pregnant women, their mean age, specimen source, HPV DNA types, HPV DNA detection methods and prevalence. Data extraction was conducted independently by two authors (P.L., Y.S.), and consensus was reached on all items. The study quality was assessed using the criteria for non-randomized observational studies (see Appendix) [Reference Duckitt and Harrington27, Reference Wei28].
Statistical analysis
We used the prevalence rate and odds ratio (OR) with their 95% confidence intervals (CIs) of the HPV prevalence in pregnant women compared to that in non-pregnant women. A summary OR with 95% CI was estimated by using both fixed-effects and random-effects models. In the presence of substantial heterogeneity (I 2 > 50%), the random-effects model was adopted as the pooling method, otherwise, the fixed-effects model was used to estimate the summary OR. Meta-regression was performed to assess the potentially important covariates exerting substantial impact on between-study heterogeneity. Stratified analyses were subsequently performed with respect to the characteristics of HPV DNA detection techniques [fluorescence in situ hybridization (FISH), Southern hybridization (SH), polymerase chain reaction (PCR), ViraPap (VP)], regions (Asia, Europe, North America), and the number of detected HPV types. Publication bias was examined by visual inspection of funnel plots and then evaluated formally with Begg's adjusted rank correlation test and Egger's regression asymmetry test. ‘Leave one out’ sensitivity analysis was performed to strengthen the result of the meta-analysis. The influence of each study was evaluated by estimating the summary ORs in their absence.
Data were analysed by Review Manager 5.1.2 software (USA) and Stata v. 11.1 (StataCorp., USA). All P values were two-sided and P < 0·05 was considered significant.
RESULTS
Literature search
For HPV infection in pregnant and non-pregnant women, the primary search generated potentially 314 relevant articles in PubMed, 190 articles in Medline, 243 articles in ISI and 192 articles in the four common Chinese databases. Three hundred and eighty articles were duplicated in the databases and excluded. Four hundred and seventy-eight articles were excluded based on screening of titles and/or abstracts using eligibility criteria. Fifty-seven articles were excluded after full-text review (one duplicated report on the same study populations, 51 failing to meet criteria and five with incomplete data), four articles were identified from reference lists. Finally, 28 articles were included in the present study, which contained 13 640 pregnant women in total. Study populations were from Asia [Reference Aydin18, Reference Chan24, Reference Hong29–Reference Peng37], Europe [Reference Schneider, Hotz and Gissmann14–Reference Chang-Claude16, Reference Soares19, Reference De21, Reference Tenti23, Reference Zlatkov25, Reference Schmeink26, Reference Louvanto38, Reference Domža39], North America [Reference Hernandez-Giron6, Reference Fife17, Reference Smith20, Reference Morrison22, Reference Smith40, Reference Peng41], Australia [Reference Worda42]. The 28 studies included are shown in Fig. 1.

Fig. 1. Selection of studies for inclusion in the meta-analysis.
The prevalence of HPV infection in pregnant and age-matched non-pregnant women
Twenty-eight eligible studies provided data on 13 640 pregnant women for HPV prevalence, in which 14 studies provided data for HPV detection only in pregnant women and the other 14 studies provided data for HPV detection both in pregnant and age-matched non-pregnant women.
The HPV prevalence in pregnant women varied from 9·58% to 46·67%, with a summary estimate of 16·82% (95% CI 16·21–17·47), and it varied from 8·9% to 23·5% in age-matched non-pregnant women, with a summary estimate of 12·25% (95% CI 11·50–13·01). The difference between the summary estimates was significant (P < 0·001, Table 1). In pregnant women, the prevalence rates of HPV infection in Australia, North America, Asia, and Europe were 36·60%, 30·37%, 15·72%, and 13·19%, respectively, showing a significant difference worldwide. The difference between the groups of pregnant and non-pregnant women in North America, Asia and Europe was seen with P < 0·05 (<0·001, 0·026, and 0·038, respectively).
Table 1. Prevalence rate of HPV infection in women across region, age and individual types

HPV, human papillomavirus; CI, confidence interval.
The prevalence rates of HPV infection were 23·94%, 13·34%, and 14·79% in pregnant women aged <25 years, 25–29 years, and ⩾30 years, respectively, and prevalence rates in non-pregnant women were 18·00%, 12·08%, and 11·43% in three age groups, respectively, the differences were significant with P = 0·025, 0·039, and 0·023, respectively.
In pregnant women, the remaining most frequently identified HPV types (prevalence rate) were HPV-16 (3·86%), HPV-6 (2·45%), HPV-18 (1·80%), and HPV-11 (1·76%). The prevalence rates of different HPV types had no statistical difference in this group compared to that in the non-pregnant women group.
The HPV prevalence rates in the three trimesters were 18·20%, 14·38%, and 19·32%, and the ORs of HPV infection were 1·59 (95% CI 1·39–1·82), 1·20 (95% CI 1·08–1·34), and 1·71 (95% CI 1·55–1·90), respectively, compared to non-pregnant women (Table 2).
Table 2. Prevalence rate and the risk for HPV infection in three trimesters
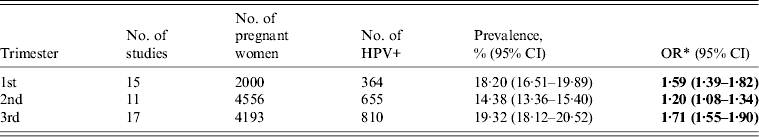
HPV, Human papillomavirus; OR, odds ratio; CI, confidence interval.
* The OR was evaluated by comparing with the HPV prevalence in non-pregnant women.
The risk of HPV infection in pregnant women
To estimate the risk of HPV infection in pregnant women, 14 studies containing both pregnant and age-matched non-pregnant women were included in the meta-analysis. Pregnant (n = 3455) and age-matched non-pregnant (n = 7190) women were enrolled, and their details are given in Table 3. The ORs for each study and overall studies can be seen in the Forest map (Fig. 2). According to the heterogeneity, the random-effects model was chosen to evaluate the summary OR. The result showed that pregnant women had a higher risk (OR 1·42, 95% CI 1·25–1·61) of HPV infection.
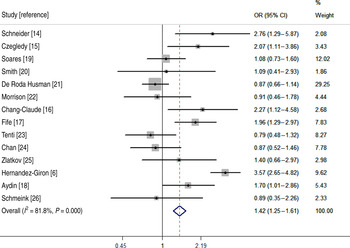
Fig. 2. Meta-analysis of odds ratio (OR) with 95% confidence interval (CI) for HPV infection in pregnant women.
Table 3. Main characteristics of the published studies included in the meta-analysis

HPV, Human papillomavirus; OR, odds ratio; SH, Southern hybridization; FISH, fluorescence in situ hybridization; VP, ViraPap; PCR, polymerase chain reaction; HC2, Hybrid Capture System II.
* Other types that were not specified in the original article.
As shown in Table 4, when pregnant women were aged <25 years, the OR was 1·79 (95% CI 1·22–2·63) for HPV infection, compared to non-pregnant women. When the ORs were pooled by region, a significantly increased HPV infection risk was shown in North America (OR 1·70, 95% CI 1·16–1·93), but this was not the case for Europe (OR 1·16, 95% CI 0·99–1·36) or Asia (OR 0·87, 95% CI 0·52–1·46). When the number of detection types was >10, there was a significantly increased HPV infection risk (OR 2·05, 95% CI 1·68–2·50) in pregnant women. There was a significantly increased risk of HPV infection in pregnant women when HPV DNA was detected using Hybrid Capture System II (HC2) (OR 3·57, 95% CI 2·65–4·82), FISH (OR 1·76, 95% CI, 1·09–2·85) and VP (OR 1·79, 95% CI, 1·16–1·93). However, there was only one study that used HC2, where the OR 3·57 (95% CI, 2·65–4·82) was not accurate. Notably, a significantly increased risk was observed in publication date periods of 1987–1999 (OR 1·19, 95% CI 1·02–1·38) and 2000–2012 (OR 2·01, 95% CI 1·62–2·50).
Table 4. Meta-analysis for stratification on the risk of HPV infection in pregnant women

HPV, Human papillomavirus; OR, odds ratio; CI, confidence interval; PCR, polymerase chain reaction; FISH, fluorescence in situ hybridization; SH, Southern hybridization; VP, ViraPap; HC2, Hybrid Capture System II.
Sensitivity analysis
To explore the heterogeneity between studies, we performed a sensitivity analysis. After omitting one study at a time and calculating the summary ORs for the remainder of the studies, the results demonstrated that there was no evidence of any individual study having an excessive influence on the summary effect (Fig. 3).

Fig. 3. Sensitivity analysis of meta-analysis for HPV infection in pregnant women.
Publication bias
The publication bias in the studies was evaluated with Egger's and Begg's tests with the results P = 0·458 and P = 0·350. The conclusion was that there was no significant publication bias and the shapes of the funnel plots (Fig. 4) did not reveal any obvious asymmetry.

Fig. 4. Begg's funnel plots for assessing the publication bias.
DISCUSSION
In this meta-analysis on the prevalence and risk of HPV infection in pregnant women, 28 studies were included and summarized. The overall HPV prevalence in pregnant women was estimated to be 16·82% (95% CI 16·21–17·47). HPV-16 was the most frequently observed type, with a prevalence of 3·86% (95% CI 3·40–4·32). The risk of HPV infection in pregnant women increased to 1·42 (95% CI 1·25–1·61).
In 2007, de Sanjosé et al. set out to estimate the prevalence of cervical HPV DNA in women with normal cytology worldwide by meta-analysis and their prevalence estimate was 10·4% (95% CI 10·2–10·7) [Reference De43]. The women in that meta-analysis represented a large age range (25–54 years) and there were no data for pregnant women. In our meta-analysis, a higher risk of HPV infection in pregnant women (OR 1·42, 95% CI 1·25–1·61) was found and the overall HPV prevalence in age-matched non-pregnant women was 12·25% (95% CI 11·50–13·01), which was comparatively higher than that in de Sanjosé et al.'s meta-analysis (10·4%, 95% CI 10·2–10·7). One of the reasons that non-pregnant women in our paper were in the optimal child-bearing period, was that there was higher sexual frequency than for non-pregnant women in de Sanjosé et al.'s meta-analysis.
Many studies have simply discussed the prevalence of HPV in different age groups in non-pregnant [Reference Clifford44–Reference Ye46] or pregnant [Reference De21, Reference Hørding47, Reference Kemp48] women. The relationship between the age of the women when pregnant and the risk of HPV infection was not considered. Fourteen studies, which provided the prevalence of HPV infection both in pregnant women and age-matched non-pregnant women, were included in our meta-analysis. In each study, the pregnant women and age-matched non-pregnant women were from the same source population and they were comparable in terms of sociodemographic variables such as age, background history of sexual activity (including number of sexual partners) and particularly, gestational age. In addition, the method for HPV detection was the same in each study. Hence, pregnant women compared to non-pregnant women in terms of HPV prevalence had little heterogeneity, and the comparisons with non-pregnant women were made feasible. This meta-analysis indicated that pregnant women who were aged <25 years had a greater risk for HPV infection.
The prevalence of HPV infection in three trimesters displayed a V-shaped trend in our study. The low HPV prevalence during the second pregnancy was partly due to HPV clearance [Reference Sarkola49]. Whereas Yamasaki et al. [Reference Yamasaki50] reported that the HPV prevalence in the third trimester was not high, this was probably related to the changes in sexual behaviour (greater stability of partnerships, less frequent intercourse), than biologically induced impacts of pregnancy upon HPV infection susceptibility and persistence. Nevertheless, data from other authors [Reference Takuwa51] showed that there were 42·9% of pregnant women newly infected by HPV between the first/second and third trimesters. Due to the clearance of virus in the first/second and third trimesters (50·4% and 71·8%, respectively), HPV prevalence remained unchanged in different periods. Hence, more research is needed to investigate the variation trend in the three trimesters of pregnancy.
Our study showed that in North America, Asia and Europe, HPV prevalence rates in pregnant women were significantly higher than those in non-pregnant women. Pregnant women in North America were more susceptible to HPV infection compared to those in Europe and Asia. In earlier investigations in Hungary and Hong Kong, HPV-16 was the most common type in asymptomatic pregnant women. The other common types were HPV-6, -18, -11, -58, -31 and -33. The sequence was a little different from that in non-pregnant women (HPV-16, -6, -11, -18, -58, -33 and -31) and other women worldwide with normal cytology (HPV-16, -18, -31, -58, -52) [Reference De43].
There was no risk of HPV infection in pregnant women when using PCR-based methods. Possible reasons are listed as follows. First, the PCR-based methods contains GP5/6 or GP5+/6+, MY09/11 or PGMY09/11, GP5/6(+) and (PG)MY09/11 combined, SPF10, HPV DNA chip, and other PCR [Reference Bruni45]. The specificity and sensitivity within PCR-based methods vary greatly, aside from built-in changes due to the development of techniques over time [Reference Bruni45]. Women from the same population tested with different techniques may double or even triple the estimated HPV prevalence [Reference Bruni45]. PCR with GP5+/6+ and PGMY09/11 showed intermediate analytical sensitivity, and PCR with SPF10 showed the highest sensitivity [Reference Snijders, van den Brule and Meijer52, Reference Gravitt53], particularly at very low concentrations of HPV, which is common in normal cytological findings [Reference Kleter54]. Another source of variability is the differential sensitivity of PCR primer sets to specific HPV types [Reference Qu55], especially with the less frequent types. The type-specific performance of the assays depends not only on the technique but also on the laboratory and the processing of the specimen [Reference Gravitt53]. Therefore, the standardization of protocols and techniques in population-based genotyping characterizations is crucial for HPV vaccine surveillance and comparisons globally.
Although the incidence of cervical cancer in pregnancy is low, about 0·02–0·4%, it is the most common tumour in pregnant women [Reference Da56]. Moreover, the published articles reported that the detection rate of infectious disease was high in pregnant women. For example, statistical data showed there was a significantly greater proportion of bacterial vaginosis (BV) in pregnant women with HPV infection compared to those without HPV infection [Reference Wang57]. At the same time, research carried by Merckx et al. [Reference Merckx12] showed there was a significantly higher risk of HPV infection in children born to HPV-positive mothers. Therefore, our results showed pregnant women had a higher risk of HPV infection, and because of the existence of maternal–neonatal transmission, we inferred that the prevalence of HPV infection in infants would be increased as well. Therefore, we suggest that pregnant women should pay more attention to limit their HPV exposure (e.g, condom use during pregnancy), in order to reduce HPV prevalence in infants.
It is necessary to consider the limitations of the present meta-analysis. First, only studies published in English and Chinese were included, which might limit the results. Second, not all pregnant women came for routine antenatal visits which made the results prone to selection bias. Third, some studies just provided the prevalence of HPV infection in one trimester, which might be higher or lower than others. This would lead to the summarized prevalence values in our review being higher or lower than the actual values. Moreover, evident heterogeneity was observed between the included studies. The results of multivariate meta-regression and stratified analyses showed that variables were not the source of heterogeneity, so further studies providing detailed information about HPV prevalence are needed to verify the current findings.
In summary, our meta-analysis suggested a significantly increased risk of HPV infection in pregnant women, especially those aged <25 years. Since potential biases and confounders could not be ruled out completely in this meta-analysis, these results need to be verified by further studies.
APPENDIX. Quality assessment of observational studies (total 10 points)

DECLARATION OF INTEREST
None.












