INTRODUCTION
Clostridium difficile is the most commonly recognized microbial cause of nosocomial diarrhoea in developed countries. The disease spectrum associated with C. difficile ranges from asymptomatic carriage to fulminant, relapsing and potentially fatal colitis [Reference Bartlett1]. Since the 1980s, several studies have reported that both the incidence of C. difficile-associated infections [Reference McDonald, Owings and Jernigan2] and the percentage of complications are increasing [Reference Drudy3–Reference Warny5], as recently indicated by the emergence of a highly virulent strain resulting in a mortality rate as high as 7% [Reference Loo6]. This C. difficile PCR ribotype 027 strain, with increased virulence has been associated with higher amounts of toxin production [Reference Warny5], has caused outbreaks in North America, Japan and Europe, and to date seven cases have been reported from Denmark [Reference Kuijper7].
Several risk factors for colonization or infection with C. difficile have been identified. Besides increasing age [Reference Asha, Tompkins and Wilcox8–Reference Raveh11], iatrogenic factors are important risk factors (e.g. antibiotic therapy, hospitalization and length of hospital stay [Reference Asha, Tompkins and Wilcox8–Reference Noren10], therapy with proton pump inhibitors [Reference Dial12, Reference Dial13] and stool softeners, gastrointestinal procedures (e.g. endoscopy, tube feeding [Reference Asha, Tompkins and Wilcox8, Reference Barbut and Petit9, Reference Schwaber14])), and the majority of episodes with gastrointestinal infection or carriage of C. difficile are considered to be nosocomially acquired [Reference Barbut15]. In healthy adults an asymptomatic carriage rate of <3% has been observed [Reference Bartlett16], although with a significantly higher asymptomatic carriage rate among children.
The immunogenic determinants of C. difficile evoking an antibody response in patients include several surface proteins [Reference Drudy3, Reference Pantosti17] and toxins (A, B and binary toxin) [Reference Warny18, Reference Viscidi19]. Moreover, antibodies, predominantly raised in rabbits, have been used in the laboratory for serogrouping and/or determination of toxin production of C. difficile, respectively [Reference Delmee, Homel and Wauters20, Reference Delmee, Mackey and Hamitou21]. In patients, antibodies against toxin A are present in both stool and serum and occur with both infection and asymptomatic carriage [Reference Warny18]. Previous studies have shown that >60% of the general population has an antibody response to toxin A or B [Reference Warny18, Reference Viscidi19], but to our knowledge no studies have yet investigated whether an increased incidence of C. difficile infection is reflected by an increased seroprevalence in the general adult population. We aimed to investigate changes in the seroprevalence of C. difficile in an adult general population followed over an 8-year period.
METHODS
Study population
The study is a part of a population-based study focused at allergic diseases in Copenhagen County. The study population was therefore selected according to a protocol aimed at investigating time trends and risk factors for allergic diseases. The protocol consisted of two parts: A baseline study in 1990 and a follow-up of the same population in 1998. The local ethical committee of Copenhagen County approved both studies.
Baseline study 1990
The baseline study in 1990 was conducted according to a two-stage protocol. In the first stage a screening questionnaire on respiratory symptoms was mailed to a sample aged 15–69 years (n=8000), living in the western part of the Copenhagen County. The sample was drawn randomly from the Civil Registration System. A total of 6998 (87·5%) responded to the screening questionnaire. In the second stage, a random group and a symptom group selected among the respondents were invited to a health examination. The random group comprised 793 subjects, who were randomly selected from the respondents. The symptom group comprised 788 subjects selected from those respondents who had reported respiratory symptoms on exposure to either pollen or furry animals in the screening questionnaire. A total of 146 subjects were included in both groups. Subsequently, 599 (participation rate 75·5%) and 635 (participation rate 80·6%) subjects were examined in the random and the symptom groups, respectively. In total 122 subjects were included in both groups. Thus, a total of 1112 subjects (overall participation rate 77·5%) were examined. Examinations took place between February 1990 and January 1991. The invitational procedure and characteristics of both participants and non-participants has previously been described [Reference Nielsen, Dirksen and Madsen22].
Follow-up study 1998
At the time of follow-up, 28 subjects had died, eight subjects had emigrated, and 12 subjects could not be located. Thus, a total of 1064 of the participants from the baseline study were invited for a re-examination; 734 subjects were examined (participation rate 69·0%). We invited eligible subjects in the same month as they were examined in the baseline study to avoid potential seasonal differences. Hence, a total of 63·9% (469/734) of the participants in the follow-up study were examined on a date within 2 months (62 days) from the date of examination in the baseline study. The median follow-up time was 7 years 10 months (range 6 years 10 months to 8 years 8 months). The participants were examined between October 1997 and November 1998. Characteristics of both participants and non-participants to the follow-up study have previously been described [Reference Linneberg23].
Serological testing
An in-house indirect ELISA measured total antibody levels in serum to C. difficile. Microtitre plates (Nunc-Immuno™ Plate, MaxiSorp™, Nunc International, Roskilde, Denmark) were coated overnight at room temperature with a 1:2000 dilution of a C. difficile antigen solution. The antigen solution was prepared by sonification of a culture of a toxigenic (toxin A and B positive) strain of C. difficile isolated from a female patient with pseudomembranous colitis. Protein concentration of the water-soluble antigen was 41·6 g/l. The antigen coating was optimized by checkerboard titration. After washing, serum samples were added in a 1:200 dilution. The assays were performed in duplicate with uncoated wells as control for non-specific binding of immunoglobulin (Ig) or conjugated anti-human Ig rabbit serum to the plastic matrix. After incubation for 1 h at room temperature the plates were washed, and horseradish peroxidase (HRP)-conjugated rabbit antibodies to human serum IgA, IgG or IgM (Dako, Glostrup, Denmark) diluted 1:2000 were added to each well. The plates were then incubated for 1 h at room temperature, washed, and the enzyme activity was detected using the orthophenylendiamine dihydrochloride (OPD)/H2O2 system. The chromogenic reaction was stopped with H2SO4 after 15–30 min and the optical density (OD) was read in a photometer at 492 nm. A difference in absorbency of >100 between coated and uncoated wells was considered positive. The Ig content was semi-quantified by serial dilution of the samples. The titres were calculated as the lowest dilution with a positive value. Seropositivity was defined as a titre >200 for C. difficile, and the cut-off value for seropositivity was identical to the values used for clinical samples.
Statistical analysis
The age of participants was categorized in groups of 8-year intervals. The prevalence of seropositivity against C. difficile was calculated for each age group in 1990 and 1998, respectively, as the proportion of seropositive persons at the time of examination in all persons in each age group.
The increase in seroprevalence by increasing age of the participants as a whole for the 1990 level was determined as the slope of the line describing seroprevalence related to age groups (mean slope of the solid line in Fig. 1). The annual increase in seropositivity during the 8-year period from 1990 to 1998 was determined as the difference in seroprevalence for the participants as a whole in 1998 and 1990 divided by 8.

Fig. 1. Seroprevalence of C. difficile in 1990 (–◆–) and 1998 (- -■- -). The seroprevalence is calculated as the proportion of seropositive persons among all persons in the age group.
The χ2 test was used for the comparison of seroprevalence between 1990 and 1998 within each age group. A regression model was set up to test, whether there was a significant increase in the overall prevalence of seropositivity to C. difficile among all participants from 1990 to 1998. The model had seropositivity to C. difficile as the binary dependent variable, and the explanatory variables were year of investigation (1990 vs. 1998), age, sex, and grouping of patients (symptom vs. random group), all binary variables except for age, which was a continuous variable. Thus, the model was:
The generalized estimation equation option was used to take repeated measurements into account. To investigate whether the symptom stratified design had influenced the results, in further analyses, data from the symptom group and random group were analysed separately. All analyses were performed with SAS software version 9.1 (SAS Institute Inc., Cary, NC, USA). P values <0·05 were considered statistically significant.
RESULTS
Seroprevalence against C. difficile
The prevalence of seropositive persons aged >15 years increased significantly over time and was 19% (210/1101) in 1990 and 27% (196/727) in 1998 (χ2, P=0·004). Seroconversion (negative in 1990 and positive in 1998, respectively) of C. difficile seropositivity occurred in 103 persons between 1990 and 1998, whereas sero-reversion (positive in 1990 and negative in 1998, respectively) of C. difficile seropositivity occurred in 48 persons.
Figure 1 shows the age-specific prevalences of seropositivity to C. difficile in 1990 and 1998. It appears that for all age groups (except for those aged 15–22 years in 1990) the age-specific prevalence of seropositivity had increased from 1990 to 1998. When analysing data for each age group separately, a significant difference in seroprevalence between 1990 and 1998 was not present in any of the age groups (see Table 1). However, when tested in the regression model the coefficient for the year of investigation, β3 (1990 level lower than 1998 level), was −0·30 (CI 95% −0·10 to −0·51) and the P value was 0·004, confirming that an overall increase in seropositivity to C. difficile had occurred from 1990 to 1998 (solid line was at all times on a lower level than the dashed line, see Fig. 1).
Table 1. Estimated seroprevalence of C. difficile in 1990 and 1998 according to age groups (% seropositive)
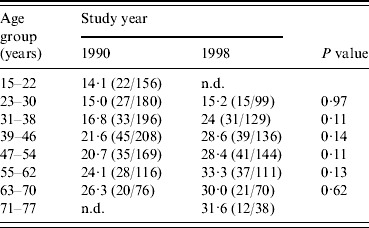
n.d., No data available.
The following calculations underline that the exposure of the population to C. difficile was higher during the period 1990–1998 than it was before 1990. From the 1990 data, which include all incident cases of seroprevalence of C. difficile in the study population between 1919 and 1990 (assuming that seropositivity persists over time), the prevalence for each extra year of living increased with about 0·2% (regression coefficient, β1=0·0172, 95% CI 0·0067–0·0277). But the increase in prevalence during the years 1990–1998 for each extra year of living was about 1% [(210/1101) – (196/727)/8] in the study population.
Seropositivity to C. difficile in 1990 was not significantly different between participants and non-participants at the follow-up in 1998 [19% (142/734) vs. 18% (68/378), respectively, P=0·58]. Moreover, the sex of participants and non-participants did not differ significantly at the follow-up in 1998 [female sex: 52% (382/734) vs. 56% (213/378), respectively, P=0·17], whereas older age was significantly associated with a lower rate of participation in follow-up in 1998 [15–34 years: 40% (296/734) vs. 37% (138/378); 35–49 years: 36% (263/734) vs. 31% (117/378); 50–69 years: 24% (175/734) vs. 33% (123/378), respectively, P=0·008].
As described above, the study population was enriched with persons with respiratory symptoms due to the symptom-stratified sampling method, which might potentially have biased the estimates for year of examination. However, in the multivariable analysis the coefficient of the indicator variable for respiratory symptoms, β4, was not statistically significant (P=0·79). Further, essentially similar results were obtained when performing separate analyses of data from persons with and without respiratory symptoms, respectively (data not shown), suggesting that the observed increase in the prevalence of C. difficile seropositivity was not due to the symptom-stratified study design.
DISCUSSION
We examined a large population-based cohort of non-hospitalized persons aged >15 years for antibodies against C. difficile over an 8-year period, and found an increase of 42% in 8 years, which cannot be explained simply by the greater age of the study population in 1998 than in 1990. Further, we observed an increase in seropositivity for all age groups supporting that there has been a higher exposure to C. difficile in Denmark between 1990 and 1998 compared to the period before 1990. Indeed, previous studies have reported an increasing incidence of C. difficile-associated diarrhoea [Reference McDonald, Owings and Jernigan2, Reference Pepin4, Reference Loo6].
Several studies have suggested that a higher immune response to C. difficile infection is associated with a lower risk of C. difficile-associated disease [Reference Drudy3, 18, 24–Reference Shim26]. Unfortunately, our study was not designed to address this important issue. Others have previously found that >60% of the general population has an antibody response to toxin A or B [Reference Warny18, Reference Viscidi19], which is higher than the seroprevalence reported here (19–27%). This discrepancy may be due to difference in methods (e.g. detection of antibodies to toxin A or B vs. antibodies to whole-cell sonicates, different cut-off values). Another limitation of our study was that no information of risk factors for Clostridium difficile-associated disease [Reference Vesta27] was recorded from the participants, and therefore we cannot exclude that our study population was indeed more often hospitalized, had an underlying disease or received antibiotics more often than the Copenhagen County population as a whole. However, the persons invited to the health examination represented a random sample of the general population. Previous comparisons of participants and non-participants in such studies have shown that participants are in general more healthy than non-participants with regard to the presence of common chronic disease.
In contrast to other studies that have reported a high median age among people with C. difficile-associated disease [Reference McDonald, Owings and Jernigan2, Reference Noren10, Reference Schwaber14, Reference Hsu28, Reference Karlstrom29]; we did not find a higher increase in prevalence in the older age groups, e.g. people aged 55–70 years in 1990 compared to people aged 47–55 years in 1990. We can only speculate on the reasons for this, but since we only measured the humeral response associated with C. difficile infection, it could be due to a combination of decreased host defence among the elderly leading to lower antibody response and our study design. In this case our results would actually underestimate the percentage of elderly people having been exposed to C. difficile, and thereby possibly also underestimate the increase in prevalence between 1990 and 1998. However, the percentages of people who underwent seroconversion in each age group were not significantly different from one another, and therefore did not actually contradict the findings from other studies.
In conclusion, we found a significant increase in seropositivity to C. difficile as reflected by detectable IgG antibodies against C. difficile in a general population. Although the increase in seropositivity may reflect both an increase in morbidity as well as an increase in asymptomatic carriers or subclinical exposure, our data overall support an increase in environmental exposure and increased immune response to C. difficile in the general Danish population.
DECLARATION OF INTEREST
None.




