Introduction
Bovine tuberculosis (bTB) is an infectious disease caused by Mycobacterium bovis, a zoonotic organism that affects cattle and many other mammals. Cattle are most likely to get infected through inhalation of aerosolised droplet nuclei [Reference Menzies and Neill1, Reference Pollock2]. Once M. bovis has entered the bronchioles/alveoli, multiplication occurs and lesions are formed [Reference Fenhalls3].
The single comparative intradermal tuberculin test (SCITT) is the main ante-mortem surveillance tool for bTB in European cattle. In Northern Ireland, all cattle over 6 weeks are tested annually with the SCITT and there is compulsory slaughter of cattle that are SCITT reactors [Reference Abernethy4]. EU legislation (European Directive 64/432/EEC (as amended)) requires post-mortem and bacteriological examination of SCITT reactors where bTB has not previously been confirmed during a bTB herd incident. In order to confirm bTB, samples from SCITT reactors identified with gross bTB-like visible lesions at post-mortem inspection are subjected to histological examination. If no histological evidence consistent with bTB are found, these samples are subjected to bacteriological culture, as are samples of bronchial and mediastinal lymph nodes from SCITT reactors with no bTB-like visible lesions [Reference O'Hagan5].
Under natural circumstances, it can take several months for infected animals to develop lesions of bTB sufficiently large to be visible at post-mortem examination. Due to this delay, the cellular immune response, which is measured by the SCITT, can be detected much earlier than gross pathology. Furthermore, the detection of lesions by visual examination at the slaughter house has been shown to be insensitive [Reference Corner6, Reference Whipple, Bolin and Miller7]. In Northern Ireland, 43% of SCITT reactors animals were found to have visible lesions considering the years 1998, 2002 and 2006. The likelihood of M. bovis confirmation in an infected animal is greatly increased by sampling from visible lesions, with 99.8% of SCITT reactors with visible lesions being confirmed by histopathology or culture, whereas only 4.3% of non-visibly lesioned SCITT reactors are confirmed by these laboratory tests [Reference O'Hagan5]. Failure to isolate M. bovis during post-mortem examination however does not necessarily mean that the animal has not been excreting the organism [Reference Menzies and Neill1].
Animals infected with environmental mycobacteria can also react positively to the SCITT, but there will normally be no evidence of bTB related visible lesions. However, the specificity of the SCITT has been estimated at over 99.9%, indicating that a SCITT false positive result is a rare event [Reference De la Rua-Domenech8, Reference Goodchild9]. The sensitivity of the SCITT shows great variation; a median of 80% (range 75–96%) at standard interpretation, highlighting the risk of residual infection in herds has been reported [Reference De la Rua-Domenech8]. Bayesian analytical techniques have suggested even lower SCITT sensitivity levels (50%) [Reference Nuñez-Garcia10].
Currently policies relating to requirements for cattle herds to regain Official Tuberculosis Free (OTF) status after a bTB incident vary across the British Isles from only requiring one negative herd-level SCITT if infection is not confirmed (termed OTS regimen; as outlined in European Directive 64/432/EEC, as amended), to requiring two consecutively negative herd-level SCITTs (termed OTW regimen) after disclosure of two or more SCITT reactors, even if no confirmation of infection is found. In Northern Ireland, at the time of writing herds with five or less unconfirmed SCITT reactors need only one clear herd-level SCITT, whereas all other incidents require two clear consecutive herd-level SCITTs at intervals of 42–60 days in order to regain OTF status.
The number of SCITT reactors is strongly correlated to the confirmation status of the bTB herd incident [Reference Karolemeas11]. A study conducted in the Republic of Ireland [Reference Olea-Popelka12], showed that the risk of a future bTB incident episode was found to increase with incident severity (as measured by grouped numbers of standard SCITT reactors) and not with the presence of (confirmed) visible lesions. These findings were later confirmed in other studies [Reference Wolfe13–Reference Clegg, Good and More16]. The current study took a different approach as it focused on the specific number of SCITT reactors during the bTB herd incident taking confirmation status into account as a risk factor for future bTB herd incident. The aim of the study was to determine an appropriate cut-off point of a number of SCITT reactors during a bTB herd incident, beyond which the OTW regimen should be applied in order for the herd to regain OTF status. The hypothesis tested was that the probability that one or more bTB-infected animals will remain in the herd after a single, negative SCITT herd test increased with an increasing number of SCITT reactors during a bTB herd incident.
Materials and methods
Study design and study population
An observational, retrospective cohort study was conducted with the study population including all new bTB herd incidents occurring during 2008 that had a 6-month follow-up herd-level SCITT Check Herd Test (CHT) after withdrawal of movement restrictions following the bTB incident. Herds that had an initial herd-level SCITT follow-up that was not a CHT were excluded from the analysis as they may have been at increased risk from other factors such as being contiguous to another bTB herd incident. New bTB herd incidents that were initiated by bTB detection in an animal at routine slaughter without any subsequent SCITT reactors were also excluded. A new bTB herd incident for the sampling frame was defined as a herd that had one or more SCITT reactors in 2008 with no SCITT reactors during the previous 12 months. Study herds were followed up for a 4-year period after regaining OTF status following the end of the initial bTB herd incident (Fig. 1). Within that 4-year follow-up period, a herd was considered to be a bTB incident again if SCITT reactors or bTB detection in animals at routine slaughter were disclosed. The methodology used for the survival study was broadly based on a study design previously used in the Republic of Ireland [Reference Olea-Popelka12].
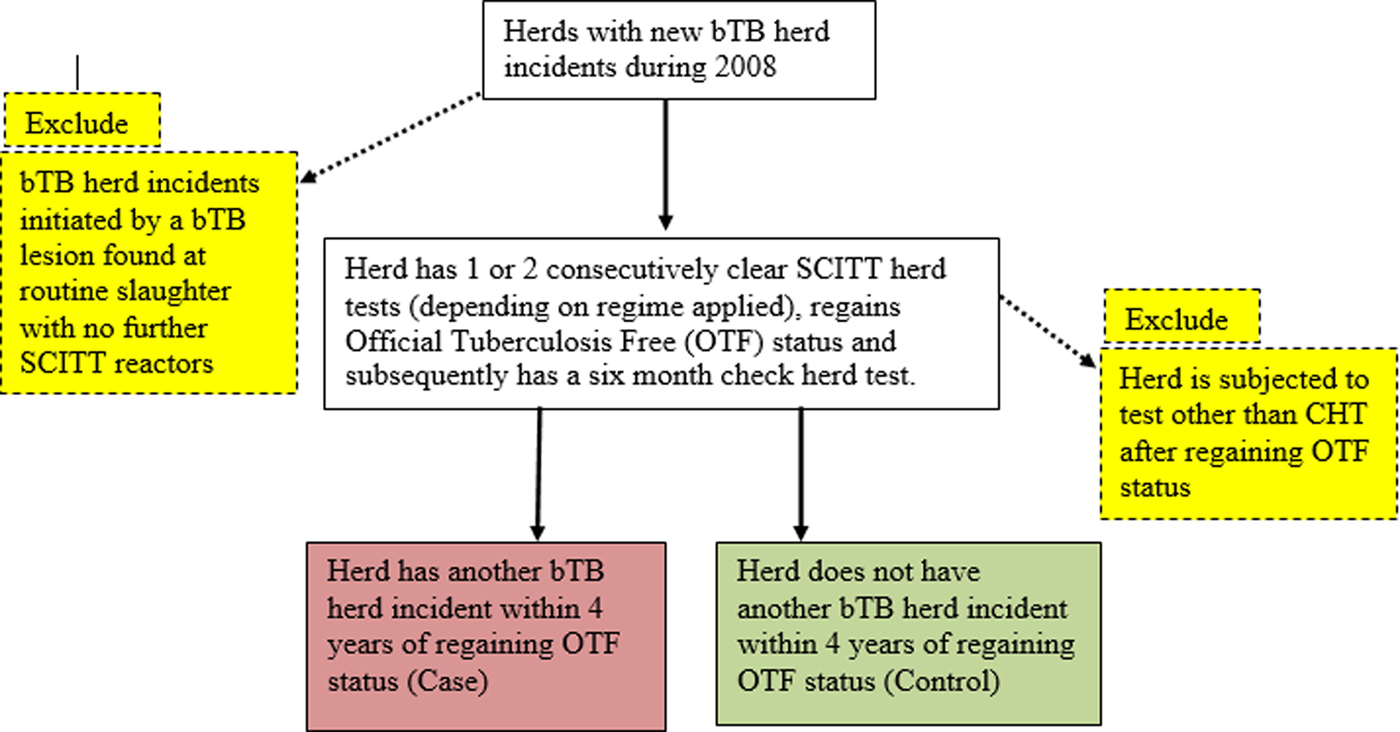
Fig. 1. Diagram outlining the study design.
Data collection and definition of variables
All data were extracted from the Animal and Public Health Information System of the Department of Agriculture, Environment and Rural Affairs. This database includes details on all individual cattle, their inter-herd movements and SCITT tests conducted since 1988 [Reference Houston17]. Datasets were merged and manipulated using MS Access™ 2007 and subsequently analysed using R 3.3.3 (the R Foundation for Statistical Computing; ‘survival’ R package [Reference Therneau18]).
Variables included in the analyses were based on characteristics of the initial bTB herd incident in 2008. They included the number of SCITT reactors (over the entire bTB herd incident period and at the disclosing SCITT), bTB confirmation, herd size, bTB history in the previous 3 years, Divisional Veterinary Office (DVO), herd type, local bTB prevalence and animal purchase intensity.
The number of SCITT reactors at the disclosing test and during the bTB herd incident was based on all animals defined as SCITT reactors with the baseline set as one SCITT reactor during the bTB herd incident. This is in contrast to other studies who compared bTB herd incidents with a baseline of herds that were clear of bTB [Reference Olea-Popelka12] or who compared herds with different categories of number of SCITT reactors during the bTB herd incident with baseline herds that had bTB detected by visible lesions in animals at routine slaughter with no further SCITT reactors [Reference Doyle15]. In the current study, the categories 1, 2, 3, 4, 5, >5 SCITT reactors were chosen in order to try and identify a justifiable cut-off point for having to implement OTW rather than OTS regimen in order to regain OTF status relating back to the current policy in the Northern Ireland bTB programme.
Confirmation of bTB infection was based on positive histology and/or bacteriological culture in samples from SCITT reactors after slaughter [Reference O'Hagan5]. Herd size was based on the average number of animals tested at herd-level SCITTs in the 3 years prior to the initial disclosure SCITT. The bTB history of the herd was a binomial variable being positive if at least one SCITT reactor (confirmed or unconfirmed) or animal with a confirmed bTB lesion at routine slaughter had been identified in the 3 years prior to the initial SCITT disclosure. Herd type was also a binomial variable (dairy/non-dairy) based on the herd possessing a milk licence. Northern Ireland is divided into 10 administrative areas called (DVO (Divisional Veterinary Office) areas, each of which is under the veterinary management of a divisional veterinary officer with smaller geographical areas or ‘patches’ that are managed by veterinary officers. The DVO area where the herd was located was included as an explanatory variable in order to adjust for regional differences. Local bTB prevalence was based on the herd prevalence in the patch area during the year that the CHT took place. The purchase intensity was based on the number of animals purchased in 90 days before either the start date of the next bTB herd incident during the follow-up period or the end date of the survival period in herds where there was no bTB herd incident during the follow-up period. This definition was similar to the definition of purchase intensity used in previous research [Reference Doyle19].
Data analyses
Survival analyses-based plots of the Kaplan–Meier estimators were conducted to evaluate the survival rate of study herds [Reference Kaplan and Meier20], focused on the number of SCITT reactors during the bTB herd incident and the bTB confirmation status of the incident. Further survival analyses were conducted on a subset of data based on bTB herd incidents that had no further SCITT reactors after the disclosure test (categorised by a number of reactors).
In order to control for potential confounders, Cox regression univariable and multi-variable models were constructed [Reference Cox and Oakes21]. Continuous explanatory variables were assessed whether they should be included in the analyses with or without categorisation, by comparing their lowess (locally weighted scatter plot smoothing) curve with a linear regression line [Reference Katz22]. If there was no significant departure of the linear regression line from the lowess curve, the explanatory variable was entered into the model as being continuous. If the variable could not be entered as a continuous variable, it was categorised using biologically appropriate cut-off points or quartiles, as appropriate.
Specifically, herd size was included as a continuous variable, purchase intensity was divided into five categories with no cattle purchases in the previous 90 days being a separate category, representing ‘closed herds’ and the remaining data being divided into quartiles. Patch bTB prevalence was divided into quartiles.
Univariable analyses were carried out on each explanatory variable and they were entered into the multivariable model if they were associated with the outcome at a P value of <0.200 using a forward stepwise method [Reference Katz22]. The best model was chosen based on Akaike information criterion (AIC) values [Reference Katz22, Reference Bradburn23]. A correlation matrix was constructed of all pairwise combinations of variables in order to assess collinearity. All combinations of two-way interactions were assessed. The linearity in the log hazard function over time was assessed by categorising the continuous variables into multiple dichotomous variables of equal units. These variables were entered into the analyses and each coefficient was graphed against the midpoint of the variable in order to assess linearity [Reference Katz22]. The proportional hazard assumption was tested using Schoenfeld residuals [Reference Grambsch and Therneau24]. The power of the study encompassing 408 events (i.e. future bTB herd incidents) was deemed to be sufficient for multivariable analyses as at a maximum 30 covariates, only 300 events are required [Reference Bradburn23, Reference Peduzzi25]. A cut-off point of P < 0.05 was considered to be statistically significant in both univariable and multivariable models.
Results
Descriptive results
There were 1036 new bTB herd incidents during 2008 that had a six-month follow-up herd-level SCITT CHT after derestriction from the bTB herd incident. Of those herds, 408 (39.4%) had a future bTB herd incident within the follow-up time.
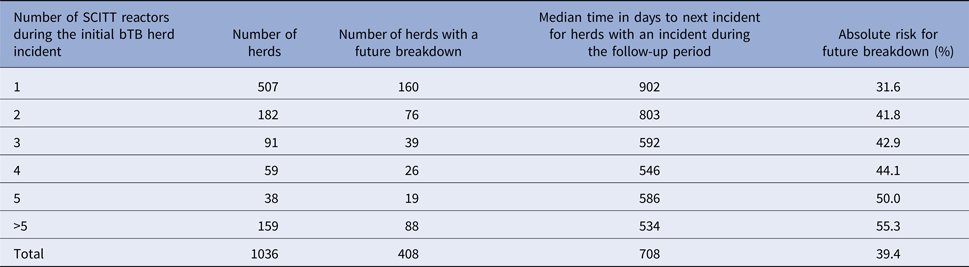
Descriptive results by a number of SCITT reactors during the initial bTB herd incident are displayed in Table 1. The absolute risk increased whereas the median time to a future incident decreased by increasing number of SCITT reactors during the initial bTB herd incident.
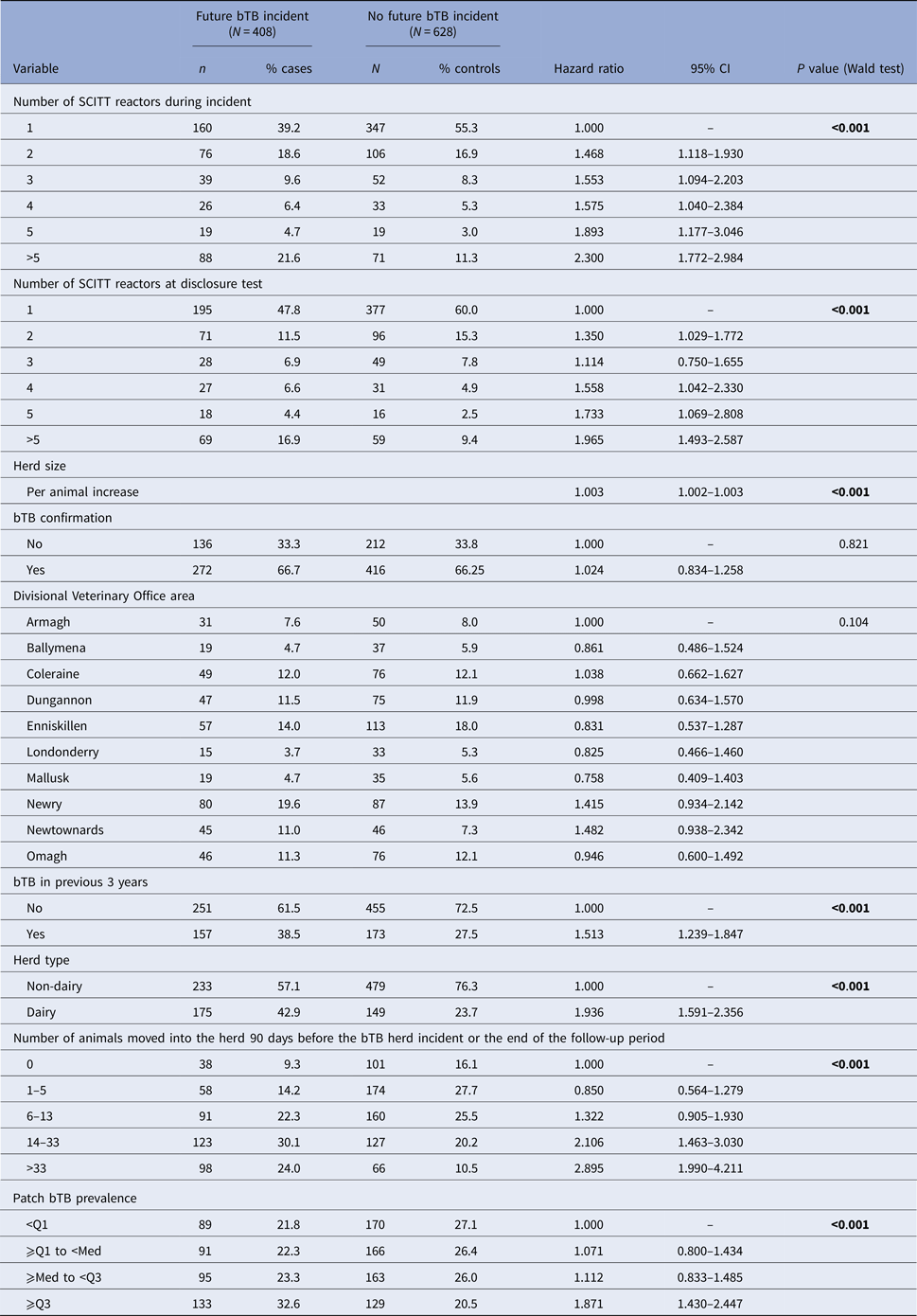
Table 1. Descriptive results in relation to future incidents by a number of SCITT reactors during the initial bTB herd incident
Survival analyses
Visual assessment of the Kaplan–Meier curves shows an increasing risk of future bTB herd incident associated with increasing number of SCITT reactors during the initial bTB herd incident (Fig. 2). Confirmation of bTB was marginally associated with an increased risk of future bTB herd incident based on this assessment (Fig. 3).
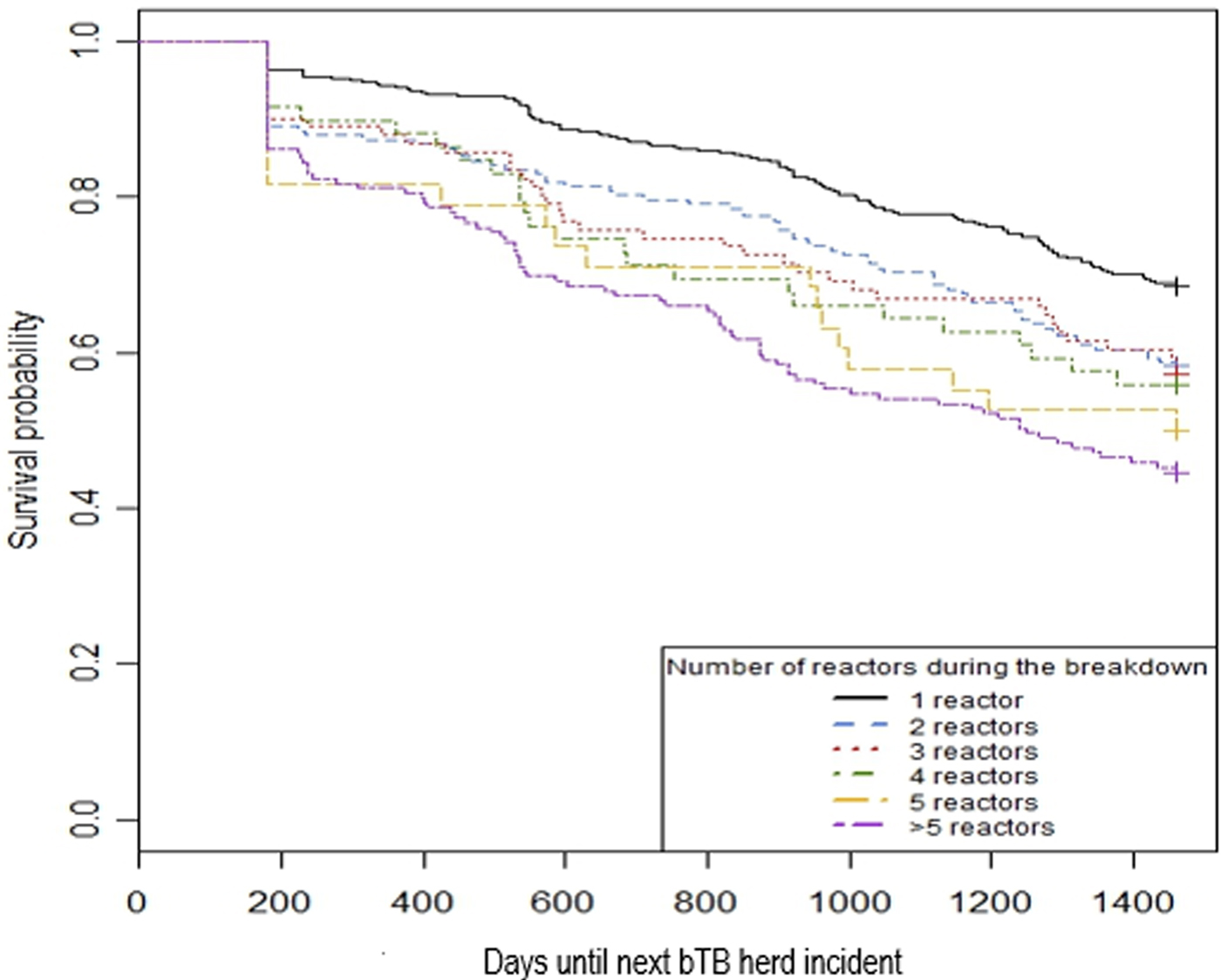
Fig. 2. Kaplan–Meier curves by a number of SCITT reactors during the initial bTB herd incident.
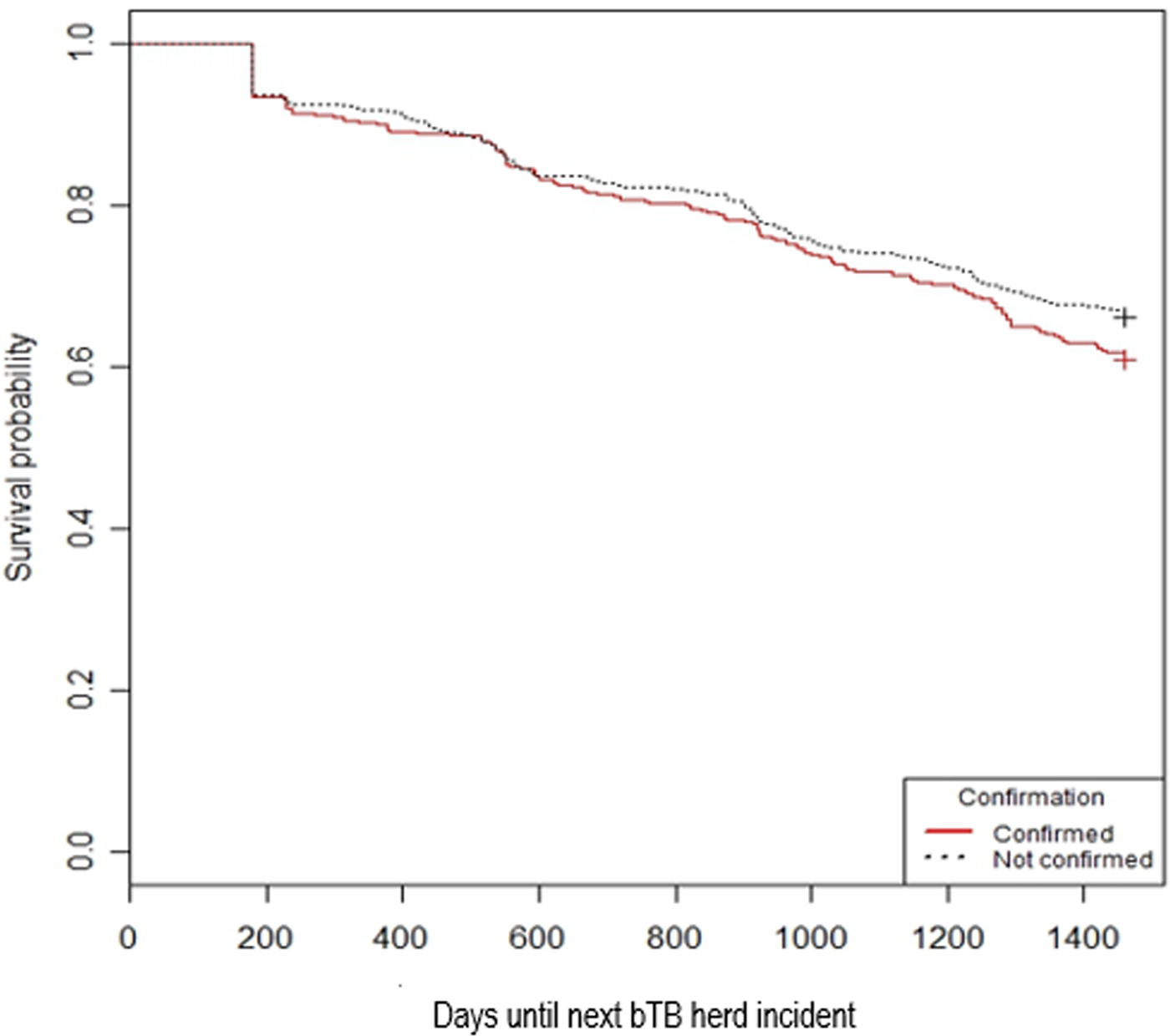
Fig. 3. Kaplan–Meier curves by bTB confirmation status during the initial bTB herd incident.
Cox regression model
Results for the univariable analyses are displayed in Table 2. Increasing number of SCITT reactors during the initial bTB herd incident and number of SCITT reactors at the disclosure test were associated with a significantly increased risk of a future bTB herd incident (hazard ratio = 2.300 (95% confidence interval (CI) 1.772–2.984) in incidents >5 SCITT reactors compared to incidents with only one SCITT reactor). Confirmation of bTB infection was not significantly associated with the risk of future bTB herd incident (hazard ratio = 1.024; 95% CI 0.834–1.258; P = 0.821).
Table 2. Univariable Cox hazard analyses of risk factors for risk of future bTB herd incident
Results of the collinearity assessment between variables showed there was a linear correlation between the number of SCITT reactors during the initial bTB herd incident and the number of SCITT reactors at the disclosure test (r = 0.68). The explanatory variable, ‘number of SCITT reactors during the bTB herd incident’, was therefore used in the multivariable model as it was considered of most biological relevance.
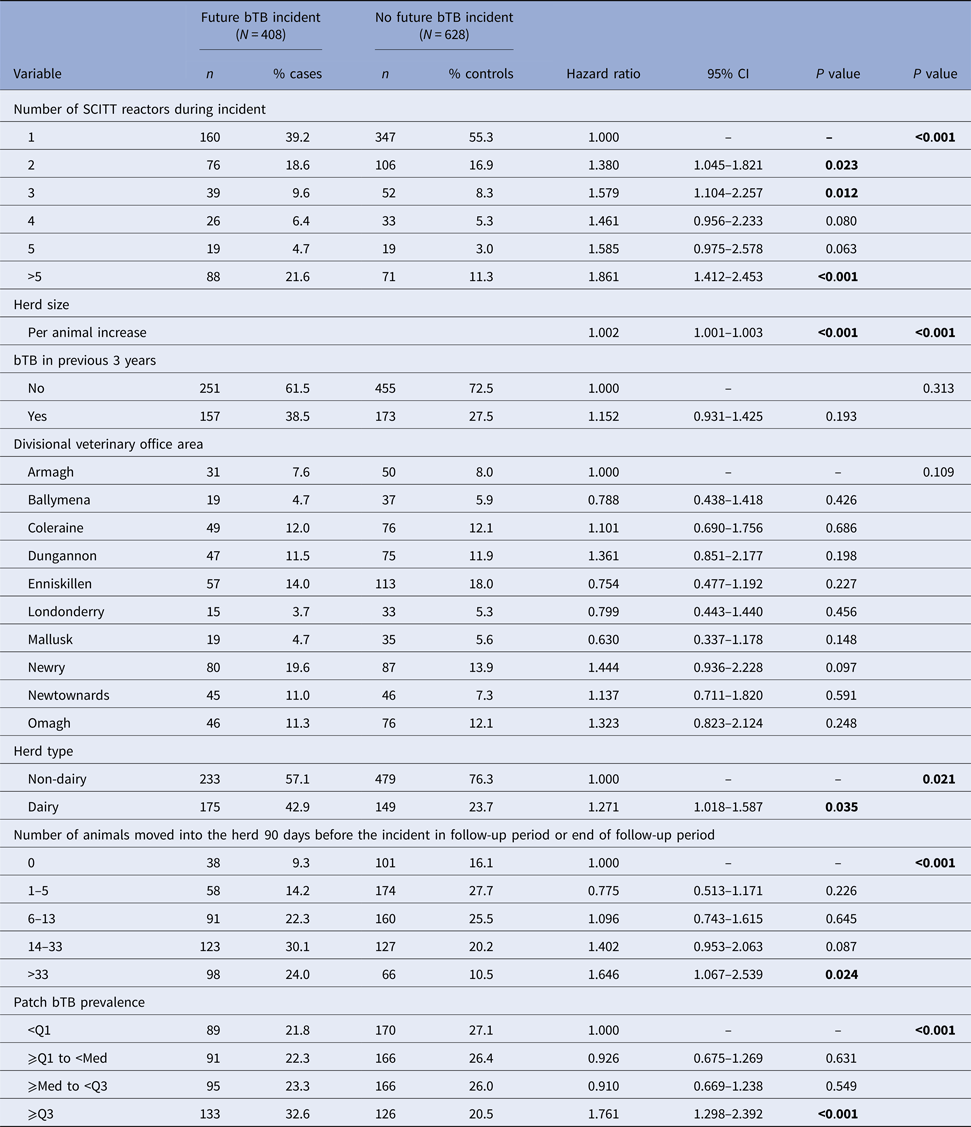
The final multivariable model (Table 3) consisted of the number of SCITT reactors during the bTB herd incident, herd size, bTB history in the previous 3 years, DVO area, herd type, purchase intensity and patch bTB prevalence. Increasing number of SCITT reactors during the bTB herd incident was associated with a significantly increased risk of a future bTB herd incident (hazard ratio = 1.861 in incidents >5 SCITT reactors compared to incidents with only one SCITT reactor; 95% CI 1.412–2.453; P < 0.001). Increasing herd size was also significantly associated with the risk of future bTB herd incident (hazard ratio = 1.002 per animal increase; 95% CI 1.001–1.003; P < 0.001). As were herd type (hazard ratio dairy herds vs. non-dairy herds = 1.271; 95% CI 1.018–1.587; P = 0.035), purchase intensity (hazard ratio = 1.646 (95% CI 1.067–2.539; P = 0.024) if >33 animals moved into the herd 90 days before the incident in the follow-up period or end of follow-up period) and patch bTB prevalence (hazard ratio = 1.761; 95% CI 1.298–2.392; P < 0.001 for upper quartile compared with lowest quartile). Schoenfeld residuals of the multivariable model showed that the proportional hazard assumption was not violated.
Table 3. Multivariable Cox hazard analyses of defined risk factors for risk of future bTB
Discussion
Overall, the incidence risk for bTB herd incident for the study herds during the 4-year follow-up was 39.4%, which was similar to the figure obtained in a study carried out in the Republic of Ireland [Reference Gallagher26]. Additionally, the 4 year risk for a future bTB herd incident almost doubled (hazard ratio = 1.861) between baseline herds (31.6%; i.e. 160/(160 + 347) × 100%) and herds with bTB herd incidents with >5 SCITT reactors (55.3%; i.e. 88/(88 + 71) × 100%) (see also Table 1). However, bTB confirmation was not predictive of the risk of future bTB herd incidents; a finding supported by several other studies [Reference Wolfe13–Reference Clegg, Good and More16] and also by the very high specificity of the SCITT [Reference De la Rua-Domenech8, Reference Nuñez-Garcia10, 27, Reference Clegg28]. The results in relation to bTB confirmation are similar to previous research conducted in Ireland [Reference Wolfe13] where in line with the current study bTB confirmation status was non-significantly associated with future bTB incidents in the univariable model and consequently left out of the multivariable model. The other risk factors for bTB recurrence were identified in the current study (herd size, herd-type, animal purchase history and local bTB prevalence) were consistent with previous research studies (reviews by [Reference Skuce, Allen and McDowell29, Reference Broughan30]).
There is variation in policy in relation to the control measures applied to bTB herd incidents with unconfirmed bTB infection between different parts of the British Isles. Whereas England's regime tends to differentiate between confirmed and unconfirmed herd incidents with regards to follow up testing except for high-risk areas [31], the Republic of Ireland makes very little differentiation and subjects herds with unconfirmed bTB herd incidents to the same follow-up regime as confirmed incidents in nearly all situations, except for herds in which only one bTB reactor is disclosed [32]. Up until 2018, the policy in Northern Ireland was that only herds with more than five SCITT reactors and unconfirmed bTB infection were subjected to the same control measures as those with confirmed incidents (OTW regimen). The main reason for evaluating this policy was to identify measures to reduce residual bTB infection in herds.
The sensitivity of the SCITT using Bayesian approaches across the British Isles estimates it to be around 50–60%, depending on different circumstances [Reference Nuñez-Garcia10, 27, Reference Clegg28] although previous reviews have suggested higher sensitivity estimates [Reference De la Rua-Domenech8]. This indicates that the sensitivity of the SCITT test is moderate at best providing ample opportunity for false negative animals to be left in bTB herd incidents if there is reliance upon one negative SCITT herd test to regain OTF status. Previous studies highlighted the importance of such residual infection in cattle herds [Reference Clegg, Good and More16, Reference Gallagher26], which can lead to recurrence [Reference Good33] alongside the costs associated with further control measures.
In addition to this, the specificity of the SCITT test is estimated to be very high [Reference De la Rua-Domenech8–Reference Nuñez-Garcia10], which would suggest that the positive predictive value of a herd with multiple SCITT reactors being truly infected with bTB approaches 100% in a country where the infection is endemic [Reference De la Rua-Domenech8]. This complements the above logic relating to residual bTB infection in herds. Furthermore, the poor sensitivity of abattoir inspection in finding gross bTB lesions alongside reported variation between abattoirs [Reference Frankena34–Reference Pascual-Linaza37] supports the findings from the current study and questions a policy where the follow-up SCITT regime after disclosure of a bTB herd incident is determined by bTB confirmation status.
The main reason why an animal is classified as an unconfirmed SCITT reactor is related to the stage of infection and the techniques employed to identify gross pathology of bTB infection. It is however thought that unconfirmed SCITT reactors could potentially be less likely to shed M. bovis than SCITT reactors with visible lesions [Reference Pollock2, Reference Pollock, Welsh and McNair38]. Nevertheless, unconfirmed SCITT reactors are known to be able to shed M. bovis [Reference Menzies and Neill1] and the issue of residual infection remains. In line with this, previous research stated that the significance of unconfirmed SCITT reactors depends on the intrinsic specificity of the screening test, the stage of the bTB eradication campaign, thoroughness of examination of reactors at slaughter, time since infection, prevalence of bTB and the number of SCITT reactors found in the herd [Reference Olea-Popelka12–Reference Doyle15]. The latter has been the focus of the current study.
Therefore it can be concluded that herds with multiple SCITT reactors should be subjected to an OTW regimen, irrespective of bTB confirmation. Introduction of this recommended policy change would give rise to greater assurances that herds are free of bTB when they regain OTF status thus limiting the inter-herd dissemination of bTB as well increasing the interval between bTB herd incidents for affected herds. In the longer term, a reduction in bTB herd incidence and overall bTB programme costs may be expected. However, further research to quantify the proportion of bTB infection in herds that is due to recrudescence of infection and that caused by re-infection from animal movements or by local spread is advocated. Nevertheless, the results of this study combined with our understanding of SCITT test performance support such a policy change in the Northern Ireland bTB eradication programme.
Conclusion
The epidemiological evidence presented here demonstrates that the risk of a future bTB herd incident increases directly with the number of SCITT reactors identified during the incident, irrespective of whether bTB infection is confirmed in the herd. The findings indicate that a policy change in relation to control of bTB in herds with multiple SCITT reactors in which bTB has not been confirmed could potentially benefit from the application of the same control measures as those applied to confirmed bTB herd incidents.
Acknowledgements
Many thanks to Mark Woodside (Department of Agriculture, Environment and Rural Affairs (DAERA)) for his assistance in relation to the data extraction, to Alan Gordon (Agri-Food and Biosciences Institute (AFBI)) for statistical advice and to Roly Harwood (DAERA) for his constructive comments on the manuscript. The authors are grateful for the comments of two anonymous reviewers which greatly improved the paper.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors have no conflicts of interest to declare.








