INTRODUCTION
Hepatitis B virus (HBV) infection and its complications remain a major worldwide health problem with more than 350 million chronically infected people and nearly one million deaths every year from acute or chronic sequelae of HBV infection, such as fulminant hepatitis, liver cirrhosis and hepatocellular carcinoma [Reference Lee1, 2]. Therefore, HBV infection is an important public health candidate for prevention, early diagnosis, and treatment [Reference Chen3]. Universal HBV vaccination is considered to be the best way to prevent HBV infection in areas with high and intermediate endemicity where the infection is predominantly transmitted at younger ages [Reference Ott4]. In 1991, the World Health Organization (WHO) recommended universal infant immunization against HBV, with a target for worldwide implementation by 1997 [Reference Alavian, Fallahian and Lankarani5, Reference Goldstein6]. As of 2008, 177 countries had implemented HBV vaccine into their national immunization programme as a routine vaccine given to all infants, which led to substantial reduction in the global burden and transmission of HBV [7].
In Tunisia, a country with an intermediate HBV endemicity, the prevalence of hepatitis B surface antigen (HBsAg) and HBV infection range from 5·3% to 7·8% and 28·5% to 48·5%, respectively [Reference Triki8, Reference Ben-Alaya-Bouafif9], with predominance of genotype D [Reference Hannachi10, Reference Meldal11]. The predominant mode of HBV transmission was the horizontal route, and HBV infection had already occurred before age 20 years especially in hyperendemic areas [Reference Ben-Alaya-Bouafif9]. HBV vaccination has been part of the Expanded Programme of Immunization (EPI) in Tunisia since 1995. During the first 10 years, the vaccination schedule consisted of three paediatric doses (10 µg recombinant HB vaccine) given at ages 3, 4 and 9 months intramuscularly in the deltoid or thigh area. Since 2006, the first administration was advanced to birth following a three-dose schedule (0, 2, 6 months). In addition, all pregnant women are screened for HBsAg and administration of post-exposure prophylaxis is indicated for infants born to HBsAg-positive women within 24 h of birth. As a result of this programme, and considering the high vaccination coverage in Tunisia (94%; Ministry of Health, Immunization coverage, April 2015) [Reference Ben Farhat12], almost all Tunisians citizens born after 1995 have been vaccinated. However, to date, there is no available local data on the epidemiological survey of HBV markers after implementation of the universal vaccination programme.
Previous worldwide data have disclosed a significant decrease in newly acquired HBV infection, carrier rate, and hepatitis B-related mortality in countries where universal vaccination has been implemented [Reference FitzSimons13, Reference Shen14]. Results of various studies revealed that 90–99% of vaccinated healthy neonates and adults develop protective levels of antibody against hepatitis B surface antigen (anti-HBs) after primary vaccination [Reference Shokri and Jafarzadeh15, Reference Qawasmi16]. However, persistence of this protective antibody was unknown and is still debated. Hence, it is important to assess if primary vaccination can confer protection until adolescence and early adulthood, when increased risky behaviour can increase the risk of contracting HBV infection [Reference Zhang17, Reference Floreani18]. Data of immunity duration in vaccinated infants are scarce, mainly in countries at intermediate and low endemicity, and requires further corroboration [Reference Schonberger19].
The objectives of the present study were to describe the change in the HBV endemicity in Tunisia for the first time 17 years after implementation of the mass hepatitis B vaccination programme, and to evaluate the efficacy and the long-term persistence of immune protection in the vaccinated Tunisian population.
MATERIALS AND METHODS
Study design and sampling
A cross-sectional, seroepidemiological survey was conducted in 2012 in the region of Sousse, located in the Eastern Centre of Tunisia. During the study period the population of Sousse was 622 100 inhabitants, of whom 171 000 (27·5%) were aged 10–25 years [20].
The targeted population included students aged 12–21 years in public colleges in the governorate of Sousse during the school year of 2012–2013. This study population constituted a representative sample of college students. Two groups were targeted: (1) vaccinated students, aged 12–17 years, born between 1995 and 2000, i.e. up to 5 years after the introduction of universal vaccination, and (2) non-vaccinated students, aged 18–21 years, born near the start of the universal HBV vaccination programme (birth years 1991–1994).
A stratified, two-stage, random cluster sampling was performed to select colleges. First, we sampled colleges, and then we sampled classes from selected colleges. Distribution of colleges in urban, suburban and rural regions was taken into account during sampling. Sample size calculation was estimated as a total of 1344 students with an expected HBV seroprevalence of 3% in the vaccinated group and 7% in the unvaccinated group. An allocation ratio between the two groups was equal to 1 (±5% level of accuracy), with a power of 90% and a confidence level of 95%.
Data collection
We assessed students’ vaccination history by examining school medical records or their personal health booklet. This booklet, which is used to record a person's vaccination history, is provided to the parents of all newborns by the hospital. According to data recorded in the health booklet, students born before 1995, who were vaccinated against HBV, were systematically excluded from the study.
In addition to collecting serum samples, vaccination dates, gender, date of birth and educational level were recorded using a structured questionnaire. Five physicians were involved with completion of questionnaires, and blood samples were obtained by four nurses.
All participants were voluntary and were informed of the study's purpose. Written informed consent was obtained from all participants or their parents for those aged <18 years.
Definitions and serological study design
HBV serum markers, including HBsAg, anti-HBs, and anti-HBc were checked in two groups according to the study's flowchart summarized in Figure 1. Anti-HBc and HBsAg were checked by microparticle enzyme immunoassay (Murex; Abbott, USA), anti-HBs was checked by Cobas e411 Elecsys analyser (Roche, USA) using original Roche kits for anti-HBs.

Fig. 1. Study flowchart.
Complete vaccination was defined as a subject receiving three doses of vaccine. Incomplete vaccination was considered when the student received only one or two doses of vaccine.
Throughout the study, we took HBsAg to be the marker of HBV infection, anti-HBs to be the marker of immunity, either from vaccination or infection, and anti-HBc to be the marker of past infection. Seronegativity was defined as being negative for all three hepatitis B markers HBsAg, anti-HBs and anti-HBc. Anti-HBs concentration ⩾10 mIU/ml was defined as protective; whereas, individuals with anti-HBs titre <10 mIU/ml were considered as non-seroprotected. The cut-off and upper detection limits for anti-HBs was 2 mIU/ml and 1000 mIU/ml, respectively. When calculating the geometric mean titre (GMT) values, a concentration of 1000 mIU/ml was used for values >1000 IU/ml.
Statistical analyses
Sample size was calculated using Epi-Info v. 6.04 (CDC, USA). Data were entered and analysed, using SPSS software v. 11.0 (SPSS Inc., USA). The results on seroprevalence and immune response to HBV vaccination are presented with point estimates and two-sided 95% confidence intervals (CIs). The outcomes for anti-HBs are expressed as seroprotective rates and GMT. χ 2 or Fisher's exact tests were used for analysing categorical data when relevant. Student's t test was used for analysing continuous data when relevant. A descriptive analysis was followed by univariate analysis using a χ 2 test or Fisher's exact test to identify factors associated with the level of long-term immunogenicity. A two-sided P value <0·05 was considered statistically significant.
Ethical standards
This study was conducted in accordance with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
RESULTS
Demography
A total of 1422 apparently healthy students aged from 12 to 21 years, from 12 colleges, were included in this study. The first group consisted of 703 vaccinated students (mean age 14·2 ± 1·1 years, male:female sex ratio 0·54). In the second group, 719 unvaccinated students were enrolled (mean age 19 ± 1 years, sex ratio 0·34) (Table 1).
Table 1. Characteristics and seroprevalence of hepatitis B markers of the study population.

Data given are number (%).
* Vaccine dose date was recorded exactly in 592 of vaccinees.
Effect of vaccination on pattern of HBV infection markers
None of the students from the two groups had positive HBsAg. The overall prevalence of anti-HBc was 0·8%. A Significantly higher prevalence of anti-HBc was showed in unvaccinated students than in those vaccinated [1·4% vs. 0·3%, odds ratio (OR) 4·944, 95% CI 1·079–22·643, P = 0·02]. The frequency distribution of the HBV markers in the groups is given in Table 1. No association was found between students’ origin and HBV markers, including anti-HBc and anti-HBs levels.
HBV vaccination history
In the vaccinated group, vaccine doses were recorded exactly in 592 (84·2%) cases, 573 (96·8%) of which had completed three doses of vaccine (Table 1). Incomplete vaccination rate was significantly higher in students from suburban areas compared to urban areas (5% vs. 1·8%, OR 2·153, 95% CI 1·185–5·651, P = 0·02).
Long-term response to HBV vaccine
Distribution of anti-HBs in vaccinated children
The overall rate of anti-HBs seroprotection in vaccinated subjects, based on a titre of anti-HBs ⩾10 mIU/ml, was 68·9% (485/703). Of these, 42% (204/485) had anti-HBs titres ⩾100 mIU/ml. An anti-HBs level of <10 mIU/ml was recorded in 31% of vaccinees (218/703) (Table 1).
Anti-HBs in different age groups
Significant difference was detected in age groups and in protective anti-HBs rates. The seroprotection rate and anti-HBs mean titre acquired through vaccination decreased significantly with age. The prevalence of protective anti-HBs titre in the 12–14 years age group (birth year 1998–2000) was 75·1% with a GMT of 154·5 mIU/ml. It decreased significantly to 60·8% with GMT values of 115·8 mIU/ml for the 15–17 years age group (birth year 1995–1997) (OR 2·231, 95% CI 1·410–2·694, P < 0·0001) (Table 2). In order to assess any trends for vaccination seroprotection in the study participants, the rate of protective anti-HBs and GMT values were assessed and stratified according to age (Fig. 2). It became evident that anti-HBs protective titre and GMT values decreased, respectively, from 78% and 130 mIU/ml for birth year 2000 (age 12 years after vaccination), to 52·4% and 38·1 mIU/ml for birth year 1995 (age 17 years after vaccination). In addition, the prevalence of students with a value <10 mIU/ml increased from 22% for birth year 2000 to 47·6% for birth year 1995. The prevalence of students featuring a serum anti-HBs level >100 mIU/ml declined from 35% for birth year 2000 to 10·4% for birth year 1995 (Fig. 3).

Fig. 2. Distribution of anti-HBs seroprotection rate and geometric mean titre (GMT) 12–17 years after vaccination in the vaccine study population.

Fig. 3. Anti-HBs levels in students born during the period from birth years 1995–2000 (12–17 years of vaccination).
Table 2. Distribution of anti-HBs seroprotection rate and geometric mean titre (GMT) by age group, gender, vaccination history and origin
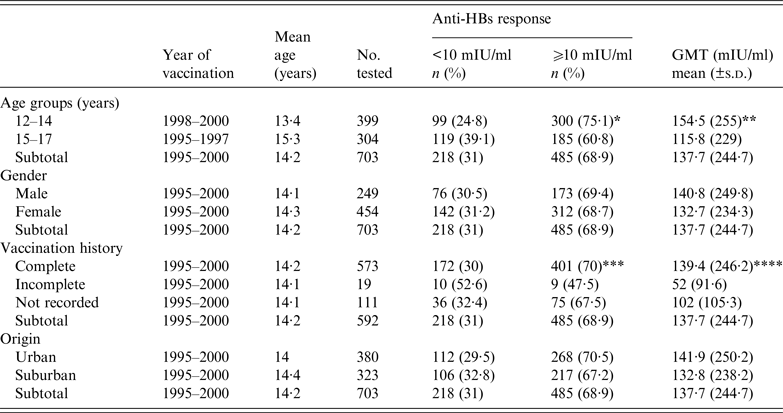
* P < 0·0001, ** P = 0·03, *** P = 0·03, **** P = 0·001 for participants with complete vs. incomplete vaccination history.
Comparative analysis of anti-HBs between males and females
No significant difference was observed in the effectiveness and protective anti-HBs levels of the HBV vaccine between males and females (Table 2).
Comparative analysis of anti-HBs between different students’ area of origin
No association was found between protective anti-HBs levels and students’ area of origin (Table 2).
Correlation with the vaccine history
The effectiveness and protective anti-HBs acquired through vaccination were significantly associated with history of vaccination. In fact, the seroprotection rate decreased significantly between subjects who received complete vaccination and those who received an incomplete course of HBV vaccine (OR 2·463, 95% CI 1·043–5·817, P = 0·03). This was supported by a decline in GMT values, from 139·4 mIU/ml in children who received complete vaccination, to 52 mIU/ml in those who received an incomplete course (P = 0·001) (Table 2).
DISCUSSION
This is the first seroepidemiological survey of HBV markers conducted in Tunisia after implementation of the universal vaccination programme in 1995. Results of this study contribute to evaluation of the impact of vaccination, providing epidemiological data of HBV infection and seroprotection over a lengthy vaccination programme (17 years).
The main results delineated a significant decrease of anti-HBc antibody, as markers of HBV infection, in students born after the implementation of the vaccination programme. In fact, comparison of results between unvaccinated and vaccinated students showed that anti-HBc-positive rates decreased from 1·4% to 0·3%. No positivity for HBsAg was detected in either of the groups.
In comparing our results to the seroepidemiological survey of HBV markers conducted in Tunisia before implementation of the universal vaccination programme, we observed a marked decline in HBV infection, attested by HBsAg and anti-HBc prevalences. In fact, in the 1995 survey, the mean prevalence rates of HBsAg and anti-HBc in the population aged between 10 and 20 years were 8% and 33% in meso-endemic areas, and 2% and 11% in hypoendemic areas, respectively [Reference Ben-Alaya-Bouafif9]. In the present survey, HBsAg was negative in all subjects aged 12–21 years, and the overall anti-HBc prevalence declined to 0·8%.
The significant decrease of anti-HBc in vaccinated students compared to those unvaccinated, and the marked decline in HBV markers in the entire group's age compared to the seroepidemiological survey conducted in Tunisia before implementation of the vaccine programme, suggest the important role of universal vaccination in the decrease of HBV endemicity.
Previous worldwide studies have also revealed the significant decrease in HBsAg carriers and anti-HBc seropositivity in children and adolescents after the mass hepatitis B vaccination programme [Reference Ang21–Reference Salleras28]. In Italy, the first Mediterranean country where mass HBV vaccination was introduced, no HBV infection was observed in vaccinated persons 17 years after vaccination [Reference Zanetti29]. In addition, a marked decline of acute HBV infection has been seen in Italy since the introduction of this mass vaccination programme [Reference Stroffolini30–Reference Banatvala, Van Damme and Oehen32].
According to the last survey conducted in Tunisia before implementation of the universal vaccination programme, a significant decrease of HBV markers was observed in all the group's ages, even in those who were not vaccinated during infancy. In fact, in the 1995 survey, the mean prevalence rates of HBsAg and anti-HBc in the population aged between 15 and 25 years were 2% and 17% in hypoendemic areas, respectively [Reference Ben-Alaya-Bouafif9]. In the present survey, HBsAg was negative in the 17–22 years age group (unvaccinated group), and anti-HBc declined to 1·4%. In light of these results, we believe that the decrease found in unvaccinated persons is connected to the decrease in HBV carriers in the area since introduction of universal vaccination, which therefore reduces the risk of contagion chain for those unvaccinated by reducing the spread of HBV in the area.
Absence of infection in this study could be attributed to the high vaccination rate (96·8%) of the three-dose HB vaccine in our study population. In 2014, the WHO strongly recommended focus on coverage of the birth dose and the three-dose vaccination regimen to control HBV infection in populations with intermediate or high HBV endemicity [7].
Other beneficial factors could contribute towards the elimination of HBV carriage. It was shown previously that urbanization plays an important role in the natural control of HBV [Reference Kew33, Reference Tsebe34]. It is therefore possible that improvement in socioeconomic status of most people in Sousse may have a positive impact on the control of HBV infection and explain the absence of positive HBsAg in our study population.
The present study showed an overall anti-HBs seroprotection rate of 68·9%, 17 years after the implementation of the universal vaccination programme. Efficacy of HBV vaccination for different age groups from several countries shows heterogeneous results. While some studies reported that 75–90% of vaccinees had protective titres of anti-HBs from 1 to 24 years after primary vaccination [Reference Chang23, Reference Coppola35, Reference Norouzirad36], in others, only 7·3–40% found this response [Reference Bialek37–Reference Zhu39]. These different data are reported in Table 3.
Table 3. Studies in different regions with different endemicity showing seroprotection after infant vaccination programme, the breakthrough infections (HBsAg, anti-HBc) and anamnestic response to a booster dose

NT, Not tested.
* Anti-HBs titre ⩾10 mIU/ml.
† An anamnestic response has defined as a rise within 10–28 days in anti-HBs concentrations to ⩾10 mIU/ml in subjects seronegative for anti-HBs antibodies (or with levels <10 mIU/ml) before challenge dose and as an increase in anti-HBs titres in subjects with seroprotective levels of anti-HBs before challenge.
Protective anti-HBs acquired through vaccination were significantly associated with history of vaccination. Indeed, the seroprotection rate is significantly lower in persons who received an incomplete course of HBV vaccine, suggesting the role of the second and third doses as boosters. The immune response rates were not associated with gender, as noted in the majority of studies [Reference Coppola35, Reference Norouzirad36, Reference Chen40], while some authors suggest a significantly better immune response for women [Reference Lim, Wong and Cheng41–Reference Morris43].
Another interesting finding of the present study is the linear decline of seroprotection rates with increasing age, from 79·3% to 52·4%, respectively, 12 and 17 years after primary vaccination. This remarkable decline in seroprotection rates was further confirmed by the significant decline in the GMT values over the past 17 years. These results were consistent with a series of studies [Reference Norouzirad36, Reference Afifi44–Reference Theeten47] reported from several countries (Table 3). A meta-analysis of 46 studies showed that the proportion of children vaccinated who retained anti-HBs concentrations >10 mIU/ml fell from 75% at age 5 years to 20% at age 20 years [Reference Schonberger19]. However, how long the effect of the immunological memory will protect vaccinated persons from future infection remains unknown.
Previous serological studies have shown that a third to half of vaccinees may have low (<10 mIU/ml) or undetectable levels of anti-HBs by ages 10–15 years [Reference Norouzirad36, Reference FitzSimons13]. In the present study, 31% had lost protective levels of antibody 12–17 years after primary vaccination. Several studies reported that individuals with waning (<10 mIU/ml) or absent concentrations of anti-HBs, long after primary vaccination, can mount a rapid and vigorous anti-HBs anamnestic response to a challenge dose of hepatitis B vaccine [Reference Salleras28, Reference Zhu39, Reference Behre45, Reference Gilca46, Reference Jafarzadeh and Montazerifar48–Reference Poovorawan50] (Table 3). These observations indicate that neonate immunization offers a lasting protection without the need for a booster dose, even when protective antibody was undetectable. Nevertheless, some studies have revealed the absence of an anamnestic response in about 20–30% of persons 20 years after primary vaccination [Reference Su22, Reference Lu51]. Moreover, cases of chronic HBV infection were reported after vaccine-induced protecting antibodies had disappeared [Reference Lu52]. However, in their latest guidelines, the WHO and the European Consensus Group on Hepatitis B Immunity do not recommend a booster dose to sustain long-term immunity in immunocompetent persons after primary immunization [7, Reference Banatvala53]. In present study, a significant decline of anti-HBs seroprotection has been observed in ⩾15-year-old adolescents with puberty-associated risky behaviours, suggesting the need of additional follow-up and testing of the anamnestic response to establish whether a primary course of vaccination in infancy may confer lifelong protection or whether boosters may be needed at this age.
On the other hand, Jack et al. suggested that anti-HBs concentration of 10 mIU/ml measured 1–3 months after administration of the last dose of the primary vaccination course is considered a reliable marker of longer protection against infection [Reference Jack54]. Subsequently, they recommended determination of anti-HBs levels in children after primary vaccination to provide long-term immunity against HBV infection.
The variability of results about seroprotective rates, long-term persistence of anti-HBs and the anamnestic response may be attributed to differences in genetic and environmental factors, type and dose of the vaccine (plasma-derived or recombinant), age of initial vaccination, schedule of immunization, body mass index and time intervals between vaccine administrations, but they may also be caused by methodological and statistical variations [Reference Su22, Reference Norouzirad36, Reference Schonberger19].
In conclusion, universal hepatitis B vaccination in Tunisia has resulted in progress towards the prevention and control of hepatitis B infection. Seventeen years after the implementation of universal vaccination, inhabitants of our region aged <21 years are protected against HBV infection. These findings should be demonstrated in other more endemic Tunisian regions. The HBV vaccination programme not only reduced the perinatal and horizontal transmission of HBV in vaccinated persons but also reduced horizontal transmission of HBV to unvaccinated persons born up to 5 years before the start of the programme. By decreasing the carrier pool, continuation of HBV immunization should prevent HBV infection in the children of Tunisia, and, subsequently, adults as well. Our data provide evidence that a strong immunological memory persists for >15 years after immunization of healthy adolescents with a primary course of hepatitis B vaccination. However, a significant decline of anti-HBs seroprotection has been observed in ⩾15-year-old adolescents with puberty-associated risky behaviours. Therefore, additional follow-up is warranted to establish whether a primary course of vaccination in infancy may confer lifelong protection or whether boosters may be needed at this age.
ACKNOWLEDGEMENTS
The authors thank the following people for their help in recruiting students and drawing blood: the team of the Department of Infectious Diseases and the team of Regional Center of Blood Transfusion, University Hospital Farhat Hached, Sousse, Tunisia. This study received no financial support.
DECLARATION OF INTEREST
None.









