INTRODUCTION
Noroviruses are responsible for a large number of infections worldwide each year. Noroviruses are highly infectious [Reference Teunis1], environmentally stable [Reference Cannon2], and able to utilize different transmission routes. Transmission can occur from person to person, after ingestion of contaminated food or water, or through contact with contaminated surfaces or aerosols [3]. Several prospective population-based studies were performed, e.g. in the UK and The Netherlands, resulting in estimates of norovirus gastroenteritis incidence of 1/80 to 1/64 of the population per annum in the UK between 1993 and 1996 [Reference Wheeler4] and 1/18 to 1/26 in 2008–2009 [Reference Tam5], and 1/31 inhabitants in The Netherlands in 1999 (Sensor) [Reference de Wit6]. The annual burden of norovirus in The Netherlands was estimated to be 450 disability-adjusted life-years (DALYs) with an incidence of 2900/100 000 (470 000 cases/year), costing Dutch society 25 million euros in 2004 [Reference Kemmeren7]. Estimating the incidence or burden due to solely foodborne norovirus transmission is difficult due to the entanglement of transmission modes; after foodborne introduction, person-to-person transmission quickly takes over.
The initial burden estimates did not include institutional norovirus outbreaks for which epidemiological and health impacts may be different [Reference Bruggink, Sameer and Marshall8]. Moreover, at the time of the Sensor study, norovirus infection was considered a mild and self-limiting disease with a low case-fatality ratio (CFR) [Reference Mead9]. Over the past decade, significant progress has been made in the field of norovirus research, yielding new knowledge about the virus and its health outcomes. For example, recent studies revealed that significant mortality may be associated with norovirus infections, particularly in the elderly [Reference van Asten10, Reference Rondy11]. Newly emerging variants have been recognized every 2 years since 2002, causing epidemics across Europe and worldwide [Reference Verhoef12] corresponding with an increase in the number of norovirus outbreaks [Reference Siebenga13] and increased mortality [Reference van Asten10].
Given the changes and new insights obtained over the last decade, there is a need for an updated burden estimate for norovirus infections. Our objective is to determine the disease burden of norovirus illness in The Netherlands in 2009 and its estimated foodborne proportion, while including the newly derived knowledge of the past decade.
METHODS
Our starting point was the burden estimate for The Netherlands 2004 [Reference Kemmeren7], using methods and updates described elsewhere [Reference Havelaar14, Reference Havelaar15].
Disease outcomes
The disease outcomes following infection were defined by designing an outcome tree, in which each block represents a health outcome, while between blocks transition probabilities must be established (Fig. 1).

Fig. 1. Outcome tree for norovirus-associated gastroenteritis.
Input parameters
The studies described in literature that provided data for our input parameters are listed in Table 1. Details of the data used are given in annex 2 of Havelaar et al. [Reference Havelaar15].
Table 1. Overview of studies providing data for the calculation of norovirus burden in The Netherlands

GE, Gastroenteritis.
Burden estimate
The different outcomes of (infectious) disease can be combined in one single metric, the DALY, following the methodology described previously [Reference Havelaar14], with a DALY being the sum of years of life lost (YLL) and the number of years lived with disability (YLD).
Community-acquired acute gastroenteritis
Age-specific incidence rates of community-acquired gastroenteritis attributed to norovirus as well as the fraction of patients visiting a general practitioner (GP) were estimated using methodology described elsewhere [Reference Havelaar14, Reference Havelaar15], using data from a nested case-control study within the 1999 population-based study Sensor [Reference de Wit16]. Information on the percentage of patients visiting their GP for a norovirus infection was derived from a nested case-control study within the Sensor study [Reference de Wit16]. These estimates were applied to the population age distribution in The Netherlands in 2009, as derived from Statistics Netherlands (www.cbs.nl). Incidence estimates were updated from 1999 to 2009 with a trend correction of 125%, as derived from trends in hospitalizations for viral gastroenteritis by all causes collected in the Dutch National Disease Registry for hospitalization (Prismant) with a national coverage of 88% [Reference Pelt van17]. According to this registry, 21 932 persons were admitted to hospital for gastroenteritis in 2009, 38% of them were children (aged <18 years). Data on aetiology were obtained from the GastroEnteritis Admission Study (Dutch acronym: GEops) [Reference Friesema18]. Briefly, patients admitted to six hospitals for gastroenteritis during the period May 2008– November 2009 were included in the study. Ninety-six faecal samples from children and 41 samples from adults (aged ⩾18 years) were analysed for pathogens by multiplex PCR (eight bacteria and five viruses) or microscopy (six parasites). At least one pathogen was detected in 98% of samples from children and 59% of samples from adults. Co-infections (two or more pathogens in one sample) were detected in 40% and 22% of samples from children and adults, respectively. The fraction of hospitalized cases due to acute gastroenteritis attributable to norovirus (fG), was modelled as a beta distribution also accounting for mixed infections (e.g. attributing the infection for half to norovirus if one additional pathogen was detected):
where G = number of samples tested for presence or absence of norovirus in GEops; posG(j) = number of samples from which norovirus was isolated as (j = 1) the only pathogen; (j = 2) with one other pathogen; (j = 3) with two other pathogens; w(j) = weight [w(1) = 1; w(2) = 1/2; w(3) = 1/3]; beta(a, b) = prior distribution for fG; in this case an informed prior distribution beta(0·15, 4) was used.
Mortality due to norovirus was derived from Germany's electronic surveillance system of infectious diseases, in which norovirus infection is statutorily notifiable [Reference Koch19] and thereby one of the few systems, if not the only, in Europe providing case-fatality ratios for all age groups. Local health departments follow-up each notification and complete a case-report that is transmitted, via state health departments, to the Robert Koch-Institute. Each case-form has a field for ‘death’, which should be marked if the death of the notified person is ‘causally related’ to the infection or where this, according to the information of the local health department, cannot be excluded. Age group-specific CFRs were derived from this surveillance system using the age categorization of the Sensor study, and applied to the age-specific estimates of community-acquired gastroenteritis attributed to norovirus in The Netherlands in 2009. An informed prior distribution beta(0·15, 4) was used. We adopted the life expectancy derived from the standard model life table (West model 25 and 26 for males and females), as recommended by WHO [Reference Murray20]. Disability weights were derived from a Dutch population panel, using elicitation protocols as described by Haagsma et al. [Reference Haagsma21], and presented in Table 2.
Table 2. Disability weights and duration
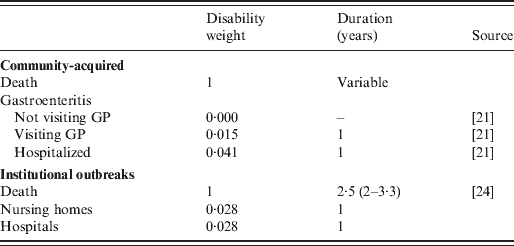
GP, General practitioner.
Institutional outbreaks
The numbers of outbreaks in nursing homes, hospitals and other institutional settings were derived from passive laboratory-based surveillance on outbreaks reported to the RIVM in 2009 [Reference Svraka22]. The mean number of cases involved in outbreaks in these settings was derived from a 1-year intensified outbreak surveillance study in The Netherlands in 2002 [Reference van Duynhoven23], while assuming that the proportion of patients visiting a GP is comparable to that in community-acquired cases. The incidence of fatal cases in institutional outbreaks (i.e. in nursing homes, hospitals and other institutional settings) was based on the case-fatality ratio for people aged ⩾65 years as derived from Germany's electronic surveillance system. For fatal cases living in institutions, a life-expectancy of 30 months was used, as described by Cunningham et al. [Reference Cunningham24], to account for comorbidity. Disability weights representative of persons living in nursing homes were not available, and may differ from the elderly living in the community due to underlying illness and quality of life. Therefore, the disability weight of living in an institution was assumed to be in the middle between the disability weight of hospital admission and visiting a GP.
Discounting
Disease burden is presented both undiscounted and discounted at a rate of 1·5% as currently recommended in The Netherlands [Reference Hakkaart-van Roijen, Tan and Bouwmans25].
Burden of foodborne disease
Community acquired
The proportion of norovirus cases attributed to food was based on expert elicitation [Reference Havelaar26], i.e. food safety experts were asked to provide their estimates of the most likely range for each of the parameters, and joint probability distributions were created by probabilistic inversion.
Outbreaks
The proportion of outbreaks attributed to food was derived from previous analyses of the Foodborne Viruses in Europe (FBVE) network's database [Reference Verhoef27].
Statistical analysis
A stochastic Monte Carlo simulation model was built to quantify the uncertainty in the disease burden of norovirus-associated illness, using @RISK 5.0 (Palisade Decision Tools, USA), a Monte Carlo simulation add-in for Excel 2002 (Microsoft, USA). The model was run for 10 000 iterations. The distribution functions of parameters that were used to estimate the disease burden of infection with norovirus are described elsewhere [Reference Havelaar15], and estimates based on new data are shown in Tables 3–5. The sensitivity of model outcomes in relation to uncertain input parameters were analysed using regression analyses using the Tornado Plot function in @RISK. Other sources of uncertainty were analysed by scenario analysis.
Table 3. Estimates of the case-fatality ratios (CFRs) based on German surveillance data 2004–2008
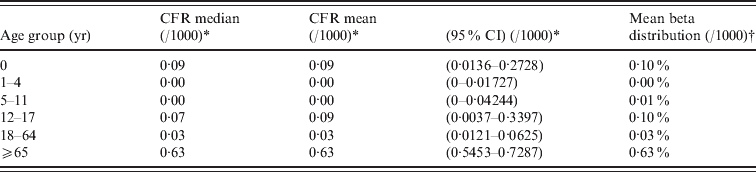
CI, Confidence interval.
* CFRs on the basis of the German surveillance system
† CFRs estimated for the Dutch population using an informed prior distribution beta(0·15, 4).
RESULTS
Community acquired
The estimates for age-specific CFRs are presented in Table 3, clearly showing the highest CFR for people aged ⩾65 years. The data of hospital admissions due to norovirus in children and adults are presented in Table 4.
Table 4. Hospitalizations due to community-acquired norovirus based on GEops data (i = 1, 2, 3) [ Reference Friesema18] as fractions of the total number of hospitalizations due to gastroenteritis in general (Prismant) [ Reference Pelt van17]
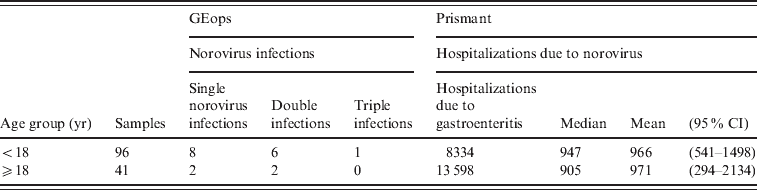
GEops, GastroEnteritis Admission Study; CI, confidence interval.
Outbreaks
The data of outbreaks in nursing homes and other settings are presented in Table 5, showing a total of 132 laboratory-reported outbreaks involving 4650 cases in The Netherlands in 2009.
Table 5. Outbreaks reported in The Netherlands, 2009 (National Institute of Public Health, The Netherlands, unpublished data), numbers of cases per outbreak [ Reference van Duynhoven23] and case-fatality ratios (CFRs) in the elderly in outbreaks [ Reference van Asten10]
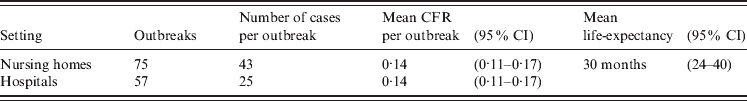
CI, Confidence interval.
Burden of disease
Community-acquired gastroenteritis
In a population of 16·5 million people the incidence of community-acquired norovirus disease cases in The Netherlands in 2009 was estimated to be 3800/100 000 (95% CI 2640–5440) of which 3700/100 000 (97·6%) (95% CI 2550–5340) were estimated as seeking no medical care, while 92/100 000 (2·4%) (95% CI 50–150) were estimated to visit a GP for their complaints, and 12/100 000 (12·5%) (95% CI 5–20) were estimated as hospitalized due to their norovirus infection. The number of fatal community-acquired cases was estimated to be 0·4/100 000 (95% CI 0·2–0·7).
Outbreaks
The number of cases involved in outbreaks in institutions was estimated to be 30/100 000, of which 20 (67%) were in nursing homes and 10 (33%) in hospitals. The number of fatal cases due to norovirus outbreaks was estimated to be 0·02/100 000.
Burden
The burden estimate calculations are shown in Tables 5 and 6. The general population incidence of norovirus gastroenteritis in 2009 was estimated to be 3800 cases/100 000 (95% CI 2670–5460), the number of fatal cases 0·4/100 000 (95% CI 0·2–0·7), the number of undiscounted DALYs 1622 (95% CI 966– 2650), and the number of discounted DALYs 1285 (95% CI 801–1910).
Table 6. Incidence of gastroenteritis due to norovirus in The Netherlands, 2009

GP, General practitioner; YLD, years lived with disability; YLL, years of life lost; DALYs, disability-adjusted life-years.
* Based on reported outbreaks, i.e. no uncertainty included.
Burden of foodborne disease
Community acquired
On the basis of expert opinion [Reference Havelaar26], 17% (95% CI 13–28) of norovirus illness cases can be attributed to food, which comprises 650/100 000 (95% CI 490–1065) cases and 0·06/100 000 (95% CI 0·05–0·11) deaths in The Netherlands in 2009, resulting in a burden of 275 (95% CI 105–450) undiscounted and 194 (95% CI 125–320) discounted DALYs.
Outbreaks
On the basis of analysis of outbreaks reported to the FBVE network [Reference Verhoef27] a total of 22% of all outbreaks can be attributed to food, which comprised 6/100 000 cases and 0·01/100 000 deaths in The Netherlands in 2009, resulting in a burden of 30 undiscounted and 30 discounted DALYs.
Overall
In 2009, a total of 662/100 000 (95% CI 496–1071) norovirus cases and 0·07/100 000 (95% CI 0·06–0·12) deaths could be attributed to food, which comprises 305 (95% CI 135–480) undiscounted and 224 (95% CI 155–350) discounted DALYs.
Sensitivity analysis
Community-acquired
The main parameters influencing the uncertainty of the overall DALY estimate, either discounted or undiscounted, were the CFR in the 12–17 years age group and 0-year-olds, and the incidence of community-acquired norovirus gastroenteritis in the 18–64 years age group and people aged ⩾65 years (data not shown). The main parameters influencing the uncertainty of deaths in community-acquired cases were incidence of community-acquired norovirus gastroenteritis in people aged ⩾65 years and, to a much lesser extent, the incidence of overall gastroenteritis in this age group. In a scenario analysis, we assumed that mortality was limited to persons that had visited a GP, as these may be considered the more severe cases. This resulted in a sharp decrease of mortality to only one fatal case and of the burden to 561 DALYs. In a second scenario, we evaluated the mortality in people aged ⩾65 years, as described by van Asten et al. [Reference van Asten10] on the basis of syndromic surveillance of unexplained gastroenteritis, i.e. a conservative estimate of 0·14 of deaths in the community for each laboratory-reported outbreak. This resulted in a total of 39 fatal cases in this age group, which is in the same order of magnitude compared to the 45 fatal cases based on the German surveillance system. In a third sensitivity scenario, we evaluated the potential effect of underreporting of mortality due to norovirus in surveillance systems, and assumed 50% of underreporting [Reference Scallan28]. This resulted in a sharp increase of mortality to 119 fatal cases an increase of the burden to 2627 DALYs.
Outbreaks
The main parameter influencing the uncertainty of the DALY estimate, either discounted or undiscounted, was the disability weight for persons living in nursing homes (regression coefficient 0·93), and can be considered a data gap.
Overall
We compared three scenarios to investigate the contribution of increased incidence and new insights into CFRs (Fig. 2) to the observed increase in the estimated burden. First, the burden of norovirus in 2004 was (re)calculated using our model but without the trend correction and using a CFR of 0·001% as described by Mead et al. [Reference Mead9]. Since Mead et al. did not provide an age stratification, 95% of the mortality was attributed to people aged ⩾65 years, and 5% to people aged <65 years. Second, the burden in 2009 was calculated using trend corrections and the CFR of Mead et al. Third, the burden was calculated using a trend correction and CFRs based on the German surveillance system but assuming no child mortality, to evaluate the influence of mortality in young children (i.e. aged <12 years). Results show that the incidence increased from 3100 to 3800/100 000 between 2004 and 2009. The corresponding increase in burden was 110 DALYs. A further increase from 900 to 1600 DALYs resulted from the new insights in mortality, of which 200 DALYs can be attributed to mortality in young children.

Fig. 2. [colour online]. Estimated burden in discounted and undiscounted disability-adjusted life-years (DALYs) while comparing the effects of different assumptions. Burden 2004: case-fatality ratio (CFR) Mead (no trend correction, CFRs as described by Mead et al. [Reference Mead9]). Burden 2009: CFR Mead (trend correction, CFRs as described by Mead et al. [Reference Mead9]). Burden 2009: CFR Germany (trend correction and CFRs as reported in the German surveillance system [Reference Koch19]). Burden 2009: CFR Germany [no child mortality; trend correction and CFRs as reported in the German surveillance system [Reference Koch19] but when setting child mortality in very young children (aged <12 years) to 0].
DISCUSSION
The burden of norovirus illness was estimated to be >1600 DALYs in The Netherlands in 2009, which is comparable to the burden of Salmonella spp. in The Netherlands [Reference Haagsma29] which, in contrast to norovirus, is well known as an enteric pathogen with a high burden. The population-based age-adjusted estimates of all norovirus cases in The Netherlands slightly increased from almost 3170 cases/100 000 in 1999 on the basis of the Sensor study [Reference de Wit6] and 3100/100 000 in 2004 [Reference Kemmeren7] to 3800 cases/100 000 in 2009 using a trend correction of 125%. However, the evidence for correction may be weak due to its indirect link to norovirus infections as a consequence of the absence of a case-based reporting system in The Netherlands. In addition, an increase was observed for one level of the reporting pyramid, i.e. hospitalizations, and there is an implicit assumption that community cases have also increased by the same proportion. Nevertheless, an actual increase is likely as a result of the emergence of new variants, as described by Siebenga et al. [Reference Siebenga13]. The updated number of 1285 estimated discounted DALYs is higher compared to ∼500 in 1999 [Reference de Wit6] and 2004 [Reference Kemmeren7]. This difference is mainly attributed to the use of a new estimate of 0·4 fatal cases/100 000 due to norovirus. The old estimate was 5 cases/100 000 in 2004, based on the CFR reported by Mead et al. [Reference Mead9], which is likely to be an underestimation. As Mead et al. explain, the assumptions underlying the Norwalk-like viruses figures were at that time among the most difficult to verify, and sensitive methods for detection were not commonly used at that time [Reference Mead9]. Moreover, different methods used for mortality estimates complicate the inferences of a time trend, as was also concluded by Scallan et al. [Reference Scallan28]. Nevertheless, higher mortality due to norovirus was found to correspond with the recent increases in norovirus activity [Reference van Asten10], which was associated with rapidly emerging new norovirus types of genogroup II type 4. The increases were either due to changes in pathogenic characteristics or a consequence of a larger number of cases including deaths, since the population is again available as a pool of susceptible persons for each new variant. The estimated mortality in children contributed considerably to the estimated DALYs: three fatal cases in children aged <5 years contributed 263 YLL (22% of the total YLL) resulting in an overall mean of 20 years of life lost per fatal case. This finding is remarkable and indicates that mortality due to norovirus needs further investigation. For deceased children laboratory testing may be more frequently performed, and thereby norovirus may be proportionally more often recognized as the causative agent, compared to other age groups. However, this would underestimate CFRs in adults instead of overestimate CFRs in children. Several groups indicated a likely underreporting of mortality rates due to norovirus for specific age groups [Reference van Asten10, Reference Harris30]. We considered the use of a surveillance system of a country where norovirus is notifiable to be the most direct approach for obtaining the CFRs for all age groups. On the basis of previous lower estimates, norovirus infection already outnumbered by far, with respect to incidence, any other foodborne pathogen [Reference Kemmeren7]. Here, we found that over 100 000 symptomatic infections and 11 deaths can be attributed to the foodborne transmission of norovirus.
Since scenario analysis showed comparable results when using mortality data from different surveillance systems, i.e. as described by van Asten et al. [Reference van Asten10], we consider this as a confirmation of the robustness of our analysis. Moreover, the CFR for outbreaks in nursing homes based on German surveillance data is in the same order of magnitude as the 0·03% found in Australia [Reference Kirk31]. In line with other burden studies, we only partly accounted for comorbidity, which may be considered a limitation of our study and may have resulted in an overestimation of the burden. However, for several reasons, we consider our estimate conservative. First, we consider underreporting of mortality due to norovirus illness likely. Underreporting is a common problem in surveillance systems [Reference Doyle, Glynn and Groseclose32], as was also illustrated during an outbreak investigation where death certificates were analysed [Reference Rondy11], and therefore the mortality ratios derived from surveillance systems may be considered conservative. Another reason is that we now assumed that every institutional outbreak was reported, which is not likely to be the case.
Sensitivity analysis also pointed out that the disability weight of disease in those living in a nursing home, the incidence of norovirus gastroenteritis in adults and the elderly, and mortality due to norovirus in young people were the main factors influencing the uncertainty in the burden estimates, and these may be data gaps to be filled by future research that can contribute to improving the burden estimates. The uncertainty in incidences is mainly due to low numbers of persons in these categories in the Sensor study [Reference de Wit6]. Given that the Sensor study was performed over a decade ago, the incidence of norovirus infections may have increased since 1999 due to newly emerging variants. For example, the studies in the UK suggest increased incidence over a 10-year period from 12–16/1000 to 38–55/1000 person-years. This potential increase is incorporated in our estimate by using updated records of hospitalizations and outbreaks. However, if a study like Sensor is performed again it may be advisable to include over-sampling of the elderly and adults, so that the uncertainties in proportions of pathogens can be diminished. The effect of the disability weight of living in nursing homes can work both ways. Either the persons living in these institutions receive better care compared to the elderly living at home, resulting in a lower disability weight, or the persons that need to live in these institutions need more care resulting in a higher disability weight. Given that several studies were performed in nursing homes in The Netherlands [Reference Friesema33, Reference Enserink34], there should be possibilities to investigate quality of life in nursing homes as well as mortality during outbreaks in the near future.
Despite the new insights in sequelae of norovirus infection, only mortality was of influence at the population level and is included in our calculations. For other sequelae, like longer duration of illness for children or hospitalized patients [Reference Lopman35], the added burden was estimated to be low, as it would not implicate chronic effects. Benign infantile seizures [Reference Chen36] are severe sequelae but have a very short duration of several minutes and no lingering symptoms. Encephalopathy [Reference Ito37] was not included because this was only described in case reports. Although irritable bowel syndrome was prospectively identified as a lingering symptom of viral gastroenteritis [Reference Marshall38], the attribution of this disease outcome to a norovirus infection is not yet established and needs further investigation. Similarly, the potential of chronic norovirus diarrhoea in immunocompromised individuals requires confirmation before it can be included in our estimates.
In conclusion, on the basis of newly gained insights in the potential severe outcome of the disease, the burden of norovirus infections overall and the consequential burden of foodborne norovirus infections are now estimated to be higher than previously assumed, despite the fact that it is still considered a conservative estimate. Several investigations illustrate the previous underestimation of the burden of norovirus illness [Reference O'Brien, Koopmans, Cliver and Bosch39], especially the foodborne proportion of norovirus infections. Still, there are knowledge gaps in the potential sequelae which need to be further investigated, and which may result in an even higher burden of norovirus illness.
ACKNOWLEDGEMENTS
We are grateful to Helen Bernard and Klaus Stark for kindly sharing the data recorded in the German surveillance system. We thank Ingrid Friesema, Jolanda Bogerman, Kees van den Wijngaard, Remko Enserink, and Yvonne van Duynhoven for their valuable feedback during the analysis. This work was supported by the Dutch Food and Consumer Product Safety Authority.
DECLARATION OF INTEREST
None.










