INTRODUCTION
Few epidemiological studies on acute encephalitis are population-based [Reference Mailles and Stahl1–Reference Glaser3] because the syndrome is rare and it may be related to several infectious causes. For several years published studies were based mainly on national hospital discharge databases to define the disease burden and give an overview of causes [Reference Khetsuriani, Holman and Anderson4–Reference Barbadoro9]. However, the usefulness and limitations of national databases for encephalitis surveillance had never been evaluated. Acute encephalitis is a severe clinical syndrome, resulting from an inflammation of the brain associated with neurological dysfunction [Reference Johnson10, Reference Tunkel11]. Infectious diseases are the main identified causes, but immune-mediated encephalitis has also recently been described [Reference Granerod2, Reference Dalmau12, Reference Irani13]. More than 100 different pathogens have been identified as causative agents of encephalitis, but demonstrating a causative relationship is sometimes difficult [Reference Stahl14, Reference Granerod15]. Viruses are responsible for most diagnosed aetiologies, and herpes viruses are the most commonly identified pathogens in industrialized countries. Herpes simplex virus (HSV) accounted for about 20% of total cases in recent population-based studies, followed by varicella-zoster virus (VZV) and Mycobacterium (M.) tuberculosis [Reference Mailles and Stahl1, Reference Granerod2, Reference Johnson10, Reference Whitley and Lakeman16]. Nevertheless, the epidemiology of encephalitis throughout the world is characterized by the predominance of cases of unknown origin, despite extensive laboratory investigation [Reference Mailles and Stahl1, Reference Granerod2, Reference Glaser17]. In previous decades, emergent or re-emergent infectious diseases causing encephalitis raised medical concern worldwide, especially infections due to West Nile virus (WNV) [Reference Campbell18], Nipah virus [Reference Chua19], enterovirus 71 [Reference Ooi20], Mycoplasma pneumonia [Reference Domenech21], European tick-borne encephalitis virus [Reference Kunze22], and Lacrosse virus [Reference Haddow, Bixler and Odoi23]. However, these causative agents are responsible for outbreaks, more easily identified and diagnosed than sporadic cases. According to previous studies, the global impact includes high case-fatality ratios (CFRs) (4–12%) [Reference Granerod2, Reference Huppatz8], long-term hospitalization (15–30 days on average) [Reference Mailles and Stahl1, Reference Barbadoro9] and severe sequelae such as physical, cognitive, emotional, behavioural, and social impairment [Reference Whitley and Lakeman16, Reference Clarke24, Reference Hokkanen and Launes25]. Encephalitis is probably an underestimated public health issue. Even if its incidence is low, it remains a serious clinical syndrome and some effective preventive or therapeutic interventions are available to fight against pathogens such as HSV, varicella, or measles. In France, infectious encephalitis is not mandatorily notifiable, but a few infections, responsible for encephalitis are: listeriosis, tuberculosis, measles, and rabies. The epidemiological data for infectious encephalitis is available from the national hospital discharge database [Reference Mailles, Vaillant and Stahl7] and from a prospective population-based study conducted in 2007 [Reference Mailles and Stahl1]. This study aimed to compare the data from the French national hospital discharge database (Programme de Médicalisation des Systèmes d'Information; PMSI) and from the prospective study conducted in 2007, to evaluate the reliability of PMSI as a tool to assess the trends of encephalitis in France for frequent, rare, and unknown aetiologies and their epidemiological characteristics, and for the detection of emergence or outbreaks.
METHODS
Data sources
Two hundred and fifty-three patients were enrolled in the prospective study [Reference Mailles and Stahl1]. According to the case definition, patients were aged ≥28 days, lived in mainland France, were hospitalized in public hospitals, were negative for HIV, and had remained in hospital for ≥5 days for surviving patients. The collected data included demographical and clinical features, and the causative agent when identified. The data was processed with Stata v. 11 (StataCorp., USA). The PMSI is a national exhaustive hospital discharge database implemented in 1997, which describes public and private hospital activity in France. For each hospitalization, the diagnoses are included in the database according to the World Health Organization (WHO) International Classification of Diseases codes, 10th revision, 2007 version (ICD-10-2007). Demographical data (age, sex), length of stay, hospital location, and death occurring during hospitalization are also recorded.
We selected records in the PMSI using criteria closely matching the case definition used in the prospective study:
• Patients aged ≥28 days, hospitalized in mainland France from January 1 to December 31 2007 in a public hospital.
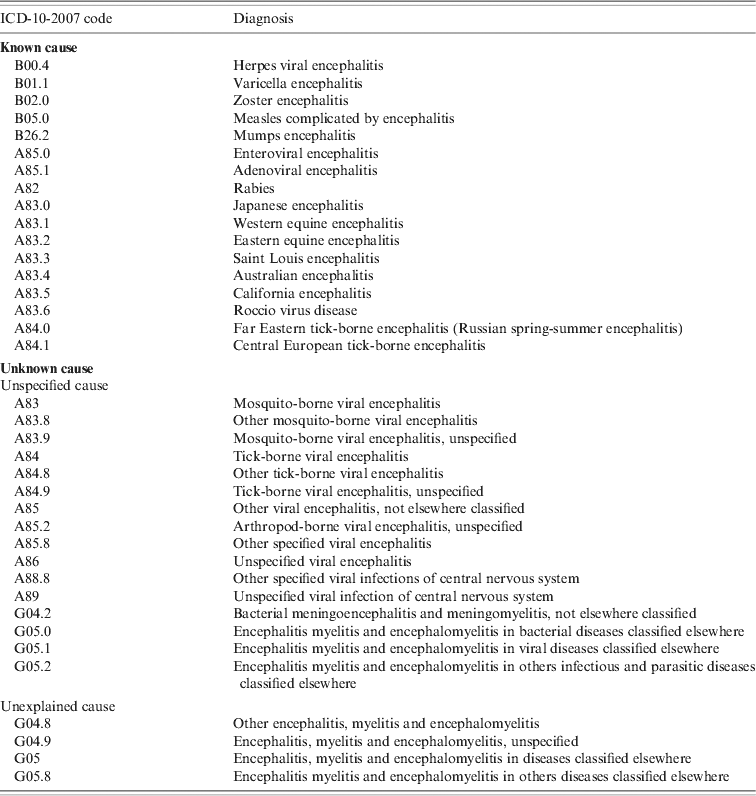
• An encephalitis-associated hospitalization was defined as a hospitalization for which at least one of the ICD-10-2007 codes for encephalitis was listed as a discharge diagnosis (main, related, or secondary). The ICD-10-2007 codes for encephalitis used to select the records are listed in Table 1.
Table 1. List of diagnostic codes in ICD-10-2007 used for extraction of encephalitis cases

Patients with multiple hospitalizations were detected using their unique identifier and only data from the first hospitalization was taken into account.
Patients matching the following criteria were excluded to maintain comparability between hospital discharge data and the prospective study:
• surviving patients with a hospital stay <5 days,
• any ICD-10-2007 code consistent with HIV infection (R75, Z21, B20–B24, F024) on the patient's file,
• codes for intracranial abscess (G06, G07), prion diseases (A810), and cerebral malaria (B500) on the patient's file.
Furthermore, if hospitalization with an unexplained or unspecified encephalitis code (Table 1) was associated with any other code consistent with encephalitis-like diseases, the patient was excluded. Toxic (G92, F10–F16, F18, F19, T40–T44) autoimmune (G35–G37, G04.0, M30–36, D86), metabolic (G40.5, E05, E10.0), vascular (G43, G45, G46, I60, I63–I68), neoplasic (C79.3, C70–C72), psychiatric (F28, F29), and congenital (G80, G60, G10–13) diseases were considered as potential encephalitis mimickers. When an encephalitis-associated code appeared in the secondary diagnosis, the patient was included if the main discharge diagnosis was related to encephalitis (compatible symptom or complication); for example, main diagnosis R40 (somnolence, stupor, and coma), and secondary diagnosis B00.4 (herpes viral encephalitis).
Incidence was calculated by using the number of inhabitants in mainland France in 2007, as estimated by the National Institute of Statistics and Economic Studies, which has responsibility for the national census.
Causes of encephalitis
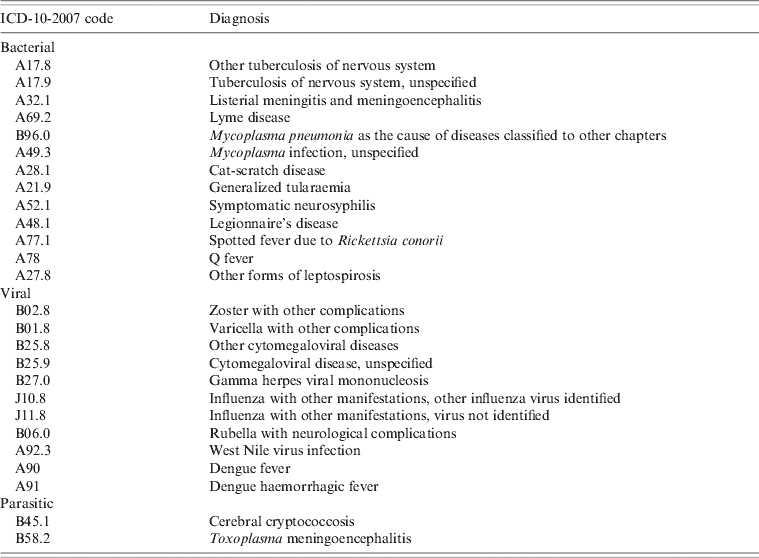
Aetiological agents were determined in the PMSI, using specific infectious encephalitis codes when defined in ICD-10-2007 (e.g. B02.0 zoster encephalitis). Some pathogens did not have any specific encephalitis code in ICD-10-2007, such as Mycoplasma pneumoniae or cytomegalovirus. In this case, the aetiological agent was kept for the diagnosis if one code listed in Table 2 (in the main, related or secondary diagnosis) was associated with a code for unspecified or unexplained encephalitis aetiology (Table 1).
Table 2. List of diagnostic codes in ICD-10-2007 used for aetiological identification when unknown causes code of encephalitis was extracted

We classified acute encephalitis hospitalizations collected from the PMSI by known cause or unknown cause and compared them with the prospective study's results.
Diagnosis in the PMSI are coded as primary diagnosis, related diagnosis (‘medical condition related to primary diagnosis’) or ‘associated’ (secondary) diagnosis (‘any medical condition that is relevant to primary diagnosis’). Within the PMSI, we compared the main characteristics of patients with primary or related diagnosis, to those of patients with secondary diagnosis.
Analysis
Encephalitis-associated cases were processed using Stata statistical software, v. 11 (StataCorp.) and sorted according to aetiology, age, gender, district of residence, duration of hospital stay, and death during hospitalization. We compared all encephalitis cases and aetiological groups in the prospective study and PMSI using two-sided t tests or non-parametric tests for continuous variables and χ2 or Fisher's exact test for categorical variables. Comparisons were assessed for statistical significance at P= 0·05.
RESULTS
Hospital discharge data (PMSI)
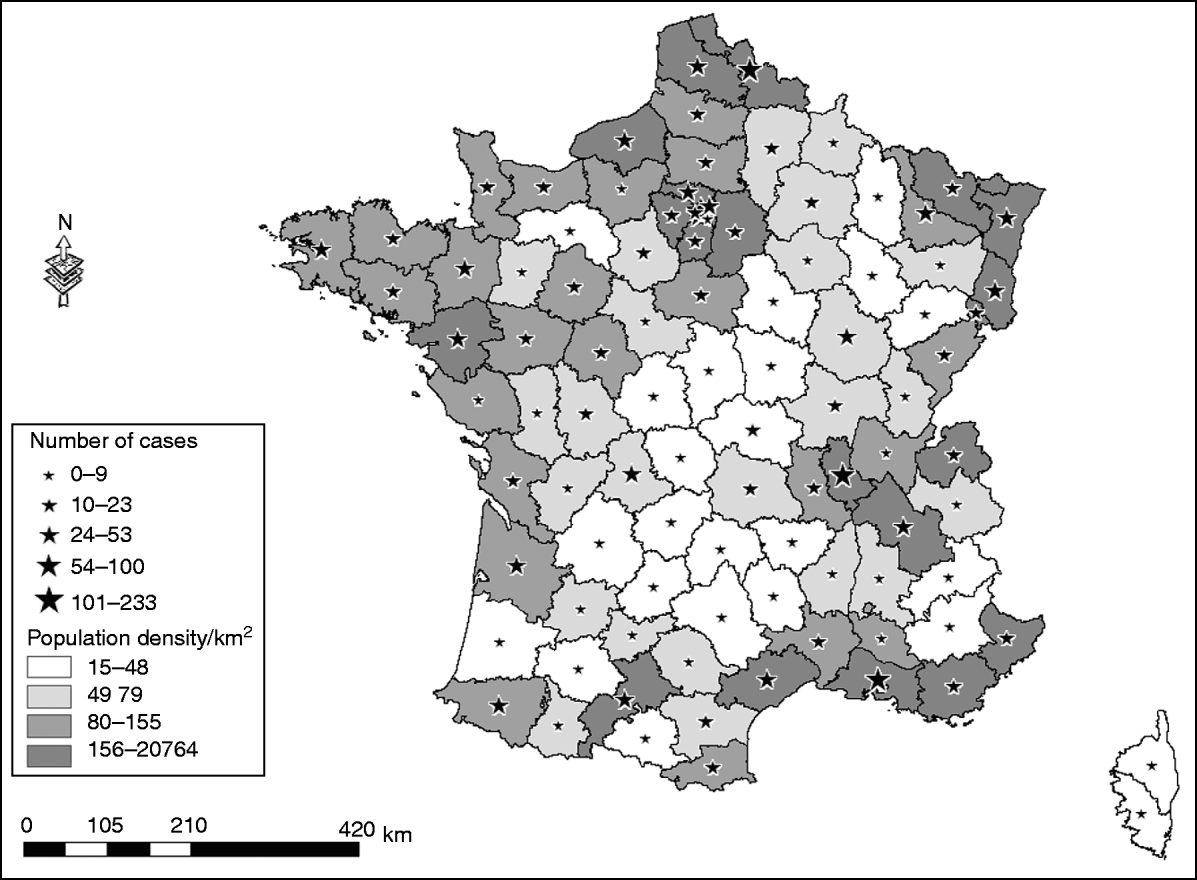
In 2007, a total of 1694 non-HIV patients presenting with acute encephalitis were recorded in the PMSI, in mainland France, making an estimated incidence of encephalitis of 2·6 cases/100000 inhabitants. The most frequently identified causes of encephalitis were HSV [320 cases (19%), 0·5 cases/100000 inhabitants], VZV [146 cases (8%), 0·2/100000 inhabitants], M. tuberculosis [30 cases (2%), 0·04/100000 inhabitants], and Listeria monocytogenes [31 cases (2%), 0·04/100 000 inhabitants]. The aetiology was unknown for 1047 (62%) patients presenting with encephalitis. The number of hospitalizations for encephalitis was higher for men (913 admissions, 54%) than for women (781 admissions, 46%), and the median age was 52 years [interquartile range (IQR) 24–71 years]. The 0–15 years age group accounted for 18% of all admissions for acute encephalitis, the median age for this group was 6 years (IQR 3–10 years). The CFR for encephalitis was 9·5% in 2007. All French districts recorded at least one case of encephalitis during that year, and the number of cases was related to the population density (Fig. 1). Overall during 2007, encephalitis patients accounted for 35557 hospital days (first hospital stay only), there was no seasonal trend by month for the main aetiological groups, for encephalitis of unknown origin and for the whole studied population.

Fig. 1. District of residence for patients presenting with encephalitis; extracted from the PMSI in France, in 2007.
For 1317/1694 patients (78%), encephalitis was the primary diagnosis and for 72 (4%) other patients, encephalitis was the ‘related’ diagnosis. For 377/1694 (22%) patients, encephalitis was an associated diagnosis. Patients with encephalitis as a primary or related diagnosis did not significantly differ from other patients (associated diagnosis) for age, sex or length of hospitalization, frequency of encephalitis due to VZV, tuberculosis and listeriosis, or for the global proportion of patients with an aetiological diagnosis. By contrast, patients significantly differed for the proportion of patients with HSV encephalitis (20% of patients with encephalitis as a primary/related diagnosis vs. 16% in patients with an associated diagnosis, P = 0·01). The CFR was significantly different with 8% death in patients with encephalitis as a primary diagnosis and 15% in patients with encephalitis as an associated diagnosis (P = 10−5).
Comparison with prospective data
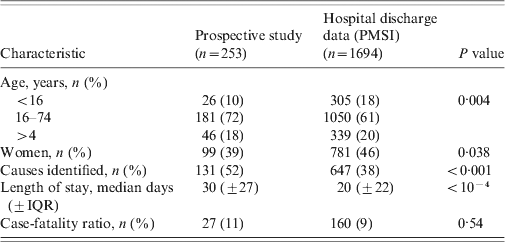
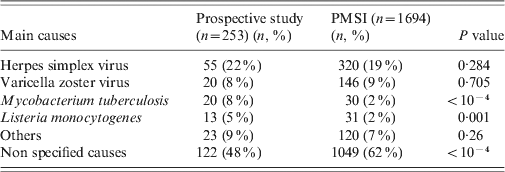
Table 3 displays the features of both populations (PMSI and prospective study) and their comparison (age, sex, age group, length of stay, proportion of known causes, CFR). No significant difference for global mean and median age was observed between the PMSI and the study populations. The number of patients in the 0–15 years age group was higher in the PMSI than in the prospective study (18% vs. 10%, P = 0·002), the proportion of encephalitis with identified aetiology was higher in the prospective study (52% vs. 38%, P < 0·001), and the mean length of stay was shorter in the PMSI (20 days vs. 30 days, P < 10−4). There was no significant difference between the PMSI and the study for the overall CFR.
Table 3. Comparison between prospective and hospital discharge data, in France, 2007

PMSI, Programme de Médicalisation des Systèmes d'Information (French national hospital discharge database); IQR, interquartile range.
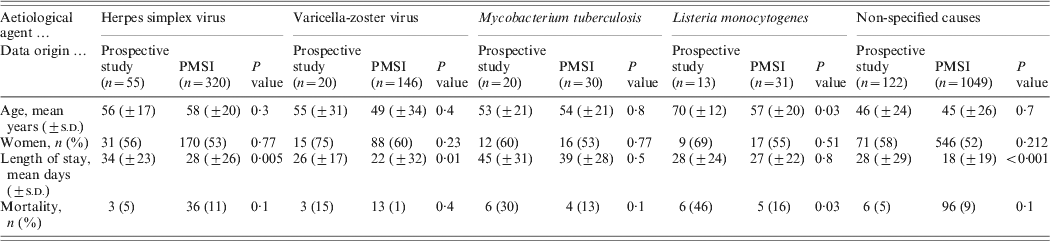
The most frequent aetiological agents associated with encephalitis were the same in both databases, nevertheless the proportion of cases of encephalitis due to M. tuberculosis and L. monocytogenes were significantly lower in the PMSI than in the prospective study (2% vs. 8%, P < 10−4 and 2% vs. 5%, P = 0·001, respectively) (Table 4). The epidemiological data was compared within each aetiological group for the main causative agents (Table 5). There was no difference for HSV and M. tuberculosis encephalitis when considering the mean age, sex, age group, length of stay, and CFR. Older mean age and higher CFR (70 vs. 57 years, P = 0·03 and 46% vs. 16%, P=0·03, respectively) was observed for L. monocytogenes encephalitis patients in the prospective study.
Table 4. Distribution of main causes of encephalitis found in the prospective study and the PMSI in France, 2007

PMSI, Programme de Médicalisation des Systèmes d'Information (French national hospital discharge database).
Table 5. Comparison of demographical characteristic and case-fatality ratio by main aetiological agent and unknown causes between the prospective study and the PMSI in France, 2007

PMSI, Programme de Médicalisation des Systèmes d'Information (French national hospital discharge database).
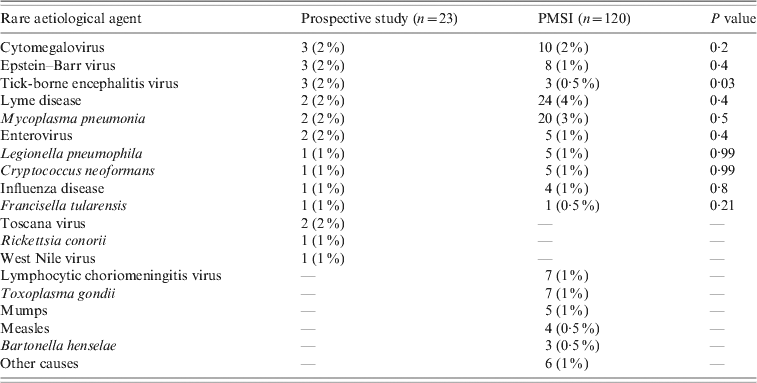
We found a higher number of patients in the 0–15 years age group and a shorter mean length of stay in the PMSI (18 vs. 28 mean days, P < 0·001) when considering encephalitis of unknown causes; but there was no difference regarding mortality. Other pathogens associated with encephalitis such as cytomegalovirus, Mycoplasma pneumonia, Borrelia burgdorferi, Epstein–Barr virus, Central European tick-borne encephalitis virus, enterovirus, Legionella pneumophila, influenza virus, Cryptococcus neoformans, and Francisella tularensis were found in both databases (Table 6). Toscana virus (TOSV), WNV and Rickettsia conorii-associated encephalitis were only reported in the prospective study. Lymphocytic choriomeningitic virus, Toxoplasma gondii, measles virus, mumps virus and Bartonella henselae-associated encephalitis were only reported in the PMSI. No comparison was made for these rare pathogens due to the small number of cases.
Table 6. Rare causes of encephalitis found in the prospective study and the PMSI in France, 2007

PMSI, Programme de Médicalisation des Systèmes d'Information (French national hospital discharge database).
Data are number (% of known causes).
Other causes included leptospirosis, pneumococcus, Japanese encephalitis, rubella, adenovirus.
DISCUSSION
The main strength of the prospective study was a single case definition and the investigation of a wide range of pathogens, but this type of study is too expensive to be conducted on a continuous basis for epidemiological surveillance. A national hospital discharge database has the advantage of being continuous, exhaustive, and available at low cost but its accuracy is limited. When compared with the multicentre prospective study [Reference Mailles and Stahl1], the encephalitis-related cases reported in PMSI in France in 2007 proved adequate for basic information. The most frequently identified causative agents were the same in both databases: HSV, VZV, M. tuberculosis and L. monocytogenes. This suggests that the PMSI may provide reliable data to study trends of encephalitis aetiologies. We found less similarity for rare pathogens such as arboviruses. The PMSI should be used with caution for these rare cases with important implications for health authorities. However, autochtonous emergence of such pathogens is likely to present as outbreaks, which are more easily detected than sporadic cases in non-endemic countries [Reference Bode26]. There is no variable that allows us to distinguish imported from autochthonous cases in the PMSI. Moreover, not all arboviral infections have a specific code in ICD-10-2007.
In the PMSI, the proportion of patients with HSV encephalitis was different according to the field of encephalitis code (20% of patient with encephalitis as a primary/related diagnosis vs. 16% in patients with associated diagnosis, P = 0·01). This small difference, although significant, could be attributed to the occurrence of herpes encephalitis in patients with current severe medical conditions: these conditions may be more economically beneficial as a primary diagnosis than encephalitis. Another explanation might be that patients with herpes encephalitis frequently present with long-term sequelae such as seizure, these syndromes then become the primary diagnosis after the acute episode.
The CFR was also significantly lower in patients with encephalitis as a primary diagnosis. Here, the most probable explanation is also the occurrence of encephalitis in patients with severe conditions (such as cancer), frequently responsible for death, and therefore more economically beneficial as a primary diagnosis.
HSV (19%) and VZV (8%) remained the main causes of acute encephalitis in both databases, as previously reported. They usually account for 13–22% and 5–6% of all published encephalitis cases, respectively [Reference Granerod2, Reference Davison5, Reference Huppatz8]. Our results suggest that the PMSI might be used for a rapid and low-cost monitoring of trends and characteristics of disease due to these pathogens. The number of deaths occurring during hospitalization in the PMSI (9%) and in the prospective study (11%) was similar for the total population and by aetiological group. We observed a difference for L. monocytogenes (16% vs. 46%, respectively) that could be explained by the inclusion of older patients in the prospective study (mean age, 57 vs. 70 years). The global CFR in the PMSI (9%) was higher than in previous retrospective studies based on national hospital discharge databases in England (6·5%) [Reference Davison5], and the USA (7%) [Reference Khetsuriani, Holman and Anderson4], and was lower than to the CFR reported in a recent prospective study in England (12%) [Reference Granerod2].
Several limitations of the PMSI as an epidemiological tool were identified in our study. One is the absence of specific codes for rare pathogens with important public health implications in ICD-10-2007. Smaller proportions were observed for M. tuberculosis and L. monocytogenes in the PMSI than in the prospective study. This probably reflects the lack of any specific code in ICD-10-2007. There is no code for tuberculous encephalitis; there is only a specific code for tuberculous meningitis (A17.0 tuberculous meningitis) and thus it was not included in our study. The misclassification of encephalitis as meningitis could explain the lower proportion of tuberculous encephalitis cases found in the PMSI. In the English prospective study, the proportion of tuberculosis was also higher (5%) than in the PMSI [Reference Granerod2] and was similar to the proportion of tuberculosis in the French prospective study, suggesting that data from the prospective study was more reliable than that of the PMSI for tuberculous encephalitis. There was no specific code that allowed us to distinguish between encephalitis and meningitis due to L. monocytogenes. The code A32.1 (Listeria meningitis and meningo-encephalitis) was not used to avoid an overestimation of encephalitis due to L. monocytogenes (see Methods section) by including meningitis cases. The cases were identified by using the association of the listeriosis code with a code for unexplained or unspecified encephalitis (Table 1). This could explain the lower number of cases found in the PMSI. The lower CFR of listeriosis is due to the fact that younger patients were coded in the PMSI. This result could be explained by the absence of a specific case definition for encephalitis due to L. monocytogenes for the PMSI, and by the broad use of the term ‘meningitis’ for all central nervous system infections due to listeriosis.
Mainland France is concerned essentially by three potentially autochthonous arboviruses that may cause encephalitis: WNV, TOSV, and Central European tick-borne encephalitis (TBE). Arboviruses became a medical concern in France in 2000 after the equine WNV epidemic in Camargue, France [Reference Murgue27]. Since then, a multispecies surveillance system has been implemented for the French Mediterranean coast region, and TOSV was added to the system in 2010. However, the occurrence of WNV autochthonous infection in France is rare and TOSV is more often responsible for meningitis than for encephalitis. For TBE there is no active surveillance because the number of TBE cases is expected to be low in France (between 5 and 10 cases per year) and its occurrence is generally limited to the eastern region (Alsace) which is the occidental limit of the European endemic zone [Reference Hansmann28]. Three cases of TBE, one of TOSV and one of WNV encephalitis were reported in the prospective study. In the PMSI, only three cases of TBE and one of Japanese encephalitis were recorded. The absence of TOSV and WNV cases in the PMSI is probably related to the absence of a specific code in ICD-10-2007. This is an important limitation when using the PMSI for the surveillance of arboviruses in France. In 2010, several European countries were confronted with a WNV epidemic (Greece, Romania, Italia) [Reference Zeller and Schuffenecker29–Reference Papa32] and ten cases of neuroinvasive TOSV infection occurred in France [33]. This emphasizes the importance of maintaining a specific surveillance system for these arboviruses in France and in Europe, and the PMSI might not be of great value for such rare pathogens. Moreover, PMSI data is not available in real time and thus does not have the reactivity required for an early detection and alerting tool.
PMSI can not be considered a real surveillance tool for several reasons, such as lack of case definition in ICD-10-2007, and the timeliness of the availability of the data. Therefore, it can only be used to assess trends of hospitalization for a disease. It can be assumed that in the near future, the improvement in information technology will speed up access to such data; however, some limitations for their use as a surveillance system will remain. First, the main objective of this database will remain economic and some questions will still arise about its reliability for epidemiology. Moreover, despite advanced technology, verification and ‘data smoothing’ procedures will still be needed, and those will have to be performed, or at least regularly checked by human beings, making the timeline still too long to achieve a reactive epidemiological surveillance.
Cases of encephalitis associated with measles were recorded only in the PMSI (n = 4). The reported incidence of this complication in the literature is 1/1000 cases [Reference Reuter and Schneider-Schlaulies34], suggesting that 4000 measles cases may have occurred in France in 2007. Measles has been mandatorily notifiable in France since 2005 and only 35 cases were notified in 2007 [35]. From 2008 to 2011, 21743 cases and 10 related deaths have been notified to the French Institute for Public Health Surveillance [35]. The cases recorded in the PMSI in 2007 might have indicated the re-emergence of the disease; nevertheless the late availability (2-year delay) of the database makes it useless to early detect any re-emergence. We note that there is a specific encephalitis code for this disease which is interesting when considering the lack of accuracy for arboviruses. Adding specific codes for arboviruses might improve the database for their surveillance.
We first chose to exclude patients with short length of stay in the study because we wanted to design a specific case definition, and avoid enrolling patients with meningitis without brain infection. In ‘real life’, some patients actually suffering encephalitis might be hospitalized for ⩽5 days without a fatal outcome, especially young children presenting with cerebellitis. However, such short length of stay is likely to be rare in France in the case of brain involvement.
The absence of a case definition of encephalitis in the PMSI is another limitation for epidemiological studies or surveillance. Mistakes in coding may occur, especially for rare pathogens not well-known by all coders. Data are primarily encoded by the attending physician. In all hospitals, a specific unit (‘medical data unit’) encompassing trained coders receives data from all wards on a regular basis (daily or weekly). This unit is responsible for verifying the completeness of data and checking for major errors (incoherent association of codes). It is also responsible for smoothing coding differences between wards and between physicians for similar cases.
Two entities rarely associated with encephalitis were found in the PMSI and appear doubtful. The first was toxoplasmic encephalitis (TE) which is a rare disease in non-immunocompromised patients. Only a few cases were reported in immunocompetent patients [Reference Habek36, Reference Kaushik37] and seven cases of TE were recorded in the PMSI without any association with codes related to HIV or any other immunodeficiency. Similarly lymphocytic choriomeningitis virus is rarely a cause of encephalitis; usually its clinical presentation is non-specific with flu-like symptoms [Reference Brouqui38]. We found seven cases in the PMSI and we suspect that it was mistakenly coded and actually lymphocytic meningitis. Another typical case is rabies; a case was recorded for a 78-year-old patient who died on the first day of hospitalization. Rabies is mandatorily notifiable in France and its specific diagnosis is only made by the Institut Pasteur. No case was notified in France in 2007. The current knowledge about rare diseases suggests some probable misclassifications. This illustrates the need to continue quality control in coding which has been implemented for a few years in order to sensitize physicians in accurate coding.
The wide range of aetiological agents explored by the prospective study can account for the smaller reported number of unknown causes compared to the PMSI. Furthermore, a diagnosis tool for rare aetiological agents may not be available in secondary- or primary-care hospitals. A retrospective study was made between 2000 and 2002 in France, using the PMSI to define encephalitis epidemiology; unknown causes accounted for 80% of all cases, which is higher than the 62% reported in the 2007 database [Reference Mailles, Vaillant and Stahl7]. The observed difference could be explained by the recent change of hospital funding in France. Indeed, since 2004 public hospital funding has depended exclusively on the coding of each hospitalization by main, related, and secondary diagnosis as defined in ICD-10-2007 [Reference Fender and Weill39]. This change probably improved the completeness and accuracy of the database. The number of unknown causes (62%) was similar to results of other retrospective studies based on national hospital discharge databases made in North America (59·5%) and Australia (69·8%), but the management and investigation of encephalitis cases could be different in other countries. Furthermore, a higher number of patients in the 0–15 years age group and a shorter mean length of stay in the PMSI was found when considering encephalitis of unknown causes. The higher number of patients in this age group in the PMSI might be explained by the presence in the PMSI of clinical syndromes such as cerebellitis, that can mimic encephalitis but have a far better outcome, or acute disseminated encephalo-myelitis (ADEM). This hypothesis is reinforced by the absence of a specific code for cerebellitis in ICD-10-2007. Moreover, only a few children were enrolled in the study which is the most probable explanation for this discrepancy [Reference Mailles and Stahl1].
The main limitation of our study is the assumption that the prospective study was representative of the global situation in France. Two main explanations can be proposed to explain the different number of cases in the two datasets. First, the prospective study included a sample of the total population of encephalitis patients because it was conducted on a voluntary basis and required a huge involvement from the investigators. The number of cases in the study is therefore an underestimation of the total number of cases of encephalitis in France in 2007. However, the similarity of our results (distribution of causative agents) with those published by Granerod et al. [Reference Granerod2] despite a different study design, suggest that the patients enrolled in our study might be representative of the global population of encephalitis patients. This is the reason why we considered the study as the ‘gold standard’ for evaluation of the PMSI.
Regarding PMSI, an overestimation of the total number of case is possible and could be explained by miscoding of the most serious cases of meningitis or by autoimmune cases (e.g. ADEM, encephalitis autoantibodies, lupus). However, a total number of 1694 cases in a year in mainland France represents an incidence of 2·6/100000 inhabitants, which is consistent with data from other countries. We are therefore confident that the discrepancy between the PMSI and the prospective study is due to a limited but representative participation in the study.
We found that cases in 0–15 years age group were under-reported in the prospective study. The PMSI might be more accurate for this age group. The authors of previous retrospective studies found that children usually accounted for 19–30% of cases, close to the 18% we identified in the PMSI. But we could not demonstrate that the adult population in the prospective study was not representative of adult encephalitis in France, and the similarities between the French and English results suggest a good representativeness of the patients enrolled in the prospective study.
CONCLUSION
This is the first study to compare these two types of data simultaneously for several aetiologies with the objective of assessing the PMSI's reliability as an epidemiological tool. The PMSI could be a useful tool for following the epidemiological trends of encephalitis of most frequent origins (HSV, VZV), as well as the characteristics of the patients and the relative frequency of these agents in encephalitis of all causes. However, the PMSI lacks accuracy and sensitivity for rare pathogens without a specific encephalitis code like M. tuberculosis or L. monocytogenes. Moreover, for rare or exotic pathogens, the absence of specific codes makes it impossible to detect their emergence and the PMSI does not allow us to distinguish between imported and autochthonous infections. Finally, the delay before database availability makes it useless for the detection of outbreaks. Our study demonstrated that data encoded in the PMSI lack accuracy about the aetiology of encephalitis, mainly due to the absence of specific codes for encephalitis due to rare infectious agents, or agents rarely responsible for encephalitis. Introducing such codes might improve both the epidemiological accuracy of the PMSI, and its accuracy as an economic tool if the severity of encephalitis, according to the aetiological agent, is taken into account.
APPENDIX
Steering committee
Cecile Bébéar (Bordeaux), Cecile Brouard (Saint-Maurice), Thomas De Broucker (Saint-Denis), Eric Cua (Nice), Henri Dabernat (Toulouse), Daniel Floret (Lyon), Benoit Guery (Lille), Marc Lecuit (Paris), Bruno Lina (Lyon), Olivier Lortholary (Paris), Alexandra Mailles (Saint-Maurice), Christian Michelet (Rennes), Patrice Morand (Grenoble), Bruno Pozzetto (Saint-Etienne), Jean-Paul Stahl (Grenoble), Veronique Vaillant (Saint-Maurice), Herve Zeller (Lyon).
Investigators
Philippe Abboud (Rouen), Chakib Alloui (Paris), Christine Archimbaud (Clermont-Ferrand), Bruno Barroso (Pau), Louis Bernard (Garches), Pascal Beuret (Roanne), Geneviève Billaud (Lyon), David Boutolleau (Paris), Fabrice Bruneel (Versailles), Marielle Buisson (Dijon), Anne Caramella (Nice), Bernard Castan (Auch), Isabelle Cattaneo (Bry sur Marne), Charles Cazanave (Bordeaux), Stéphane Chabrier (Saint-Etienne), Marie-Laure Chadenat (Versailles), Martine Chambon (Clermont-Ferrand), Pascal Chavanet (Dijon), Pierre Clavelou (Clermont-Ferrand), Eric Cua (Nice), Fabienne de Brabant (Montélimar), Arnaud De La Blanchardière (Caen), Geoffroy De La Gastine (Caen), Henri De Montclos (Bourg-en-Bresse), Eric Denes (Limoges), Anny Dewilde (Lille), Aurelien Dinh (Garches), Guillaume Emeriaud (Grenoble), Olivier Epaulard (Grenoble), Giovanni Favaretto (Avranche), François Fourrier (Lille), Véronique Gaday (Pontoise), Jacques Gaillat (Annecy), Serge Gallet (Montluçon), Nicole Gazuy (Clermont-Ferrand), Stéphanie Gouarin (Caen), Joel Gozlan (Paris), Philippe Granier (Bourg-en-Bresse), Isabelle Gueit (Rouen), Amélie Guihot (Paris), Yves Guimard (Bourges), Yves Hansmann (Strasbourg), Cécile Henquell (Clermont-Ferrand), Jean-Louis Herrmann (Garches), Jérome Honnorat (Lyon), Nadhira Houhou (Paris), Benoit Jaulhac (Strasbourg), Manoelle Kossorotoff (Paris), Frédéric Laurent (Lyon), Jean-Jacques Laurichesse (Paris), Sylvain Lavoue (Rennes), Leila Lazaro (Bayonne), Stephane Legriel (Versailles), Olivier Lesens (Clermont-Ferrand), Muriel Mace (Orléans), Alain Makinson (Montpellier), Hélène Marchandin (Montpellier), Laurent Martinez-Almoyna (Saint-Denis), Patrick Marthelet (Montélimar), Martin Martinot (Colmar), Laurence Maulin (Aix-en-Provence), Benoit Misset (Paris), Catherine Neuwirth (Dijon), Florence Nicot (Toulouse), Jérome Pacanowski (Paris), Jean-Bernard Palcoux (Clermont-Ferrand), Patricia Pavese (Grenoble), Thomas Perpoint (Lyon), Martine Pestel–Caron (Rouen), Robin Pouyau (Lyon), Virginie Prendki (Paris), Christophe Rapp (Saint-Mandé), Christel Regagnon (Clermont-Ferrand), Matthieu Rigal (Auch), Nathalie Roch (Grenoble), Olivier Rogeaux (Chambéry), Sylvie Rogez (Limoges), Anne Signori-Schmuck (Grenoble), Fabrice Simon (Marseille), Abdelilah Taimi (Roanne), Jérome Tayoro (Le Mans), Daniel Terral (Clermont-Ferrand), Francis Vuillemet (Colmar).
DECLARATION OF INTEREST
None.