Introduction
Gastric cancer is the third leading cause of cancer death in the world [1]. Japan has the third highest age-standardised incidence of gastric cancer [2]. Gastric cancer screening using upper gastrointestinal series, endoscopy and serological testing (Helicobacter pylori (H. pylori) antibody test (HPA) and serum pepsinogen screening) has been performed in population-based (employee-based and community-based) and opportunistic cancer screening in Japan. The public healthcare system of the local government also has influence over cancer screening. The upper gastrointestinal series with double-contrast study has been conducted based on the Japanese guidelines as a public policy based. However, the detection rate in gastric cancer screening has remained at 0.1–0.2% [Reference Hamashima3]. There were 45 531 gastric cancer deaths in 2016 [4]. This is a high number attributable to the low uptake of effective screening with mortality reduction from gastric cancer, as not all of these could have been prevented by screening. Radiographic and endoscopic gastric cancer screenings are recommended in update version of the Japanese guidelines for population-based and opportunistic screenings [Reference Hamashima5]. The company provides employee-based cancer screening for the employees and each health insurer offers opportunistic cancer screening in Japan [Reference Goto6]. These gastric cancer screening rates are low, too [7]. Employees are a high-risk population for gastric cancer in Japan.
H. pylori is a helix-shaped Gram-negative, microaerophilic bacterium. H. pylori infection is well known to cause peptic ulcer diseases, atrophic gastritis, gastric cancer and mucosa-associated lymphoid tissue (MALT) lymphoma. H. pylori eradication reduces gastric cancer incidence and prevents gastric cancer, as well as H. pylori-positive peptic ulcer disease and MALT lymphoma [Reference Kabir8–Reference Ford12]. H. pylori screening followed by eradication treatment is recommended in high-risk population settings to reduce gastric cancer incidence. A retrospective cohort study in Korea demonstrated that H. pylori eradication reduced the cumulative incidence of gastric cancer in healthy asymptomatic population and showed that the effect of H. pylori eradication on the prevention of gastric cancer was observed in all ages [Reference Bae13]. Lee et al. showed that population-based eradication of H. pylori infection has led to a significant reduction in gastric atrophy at the expense of increased esophagitis [Reference Lee14]. Asia-Pacific and US Gastric Cancer Consensus conference recommend H. pylori screening and treatment in asymptomatic persons from high-risk populations to prevent gastric cancer [Reference Fock15, Reference Talley, Fock and Moayyedi16].
Cost-effectiveness regarding H. pylori infection screening method followed by eradication warrants evaluation as a gastric cancer policy control measure in the occupational health setting.
In this study, cost-effectiveness of HPA screening followed by eradication treatment was assessed to evaluate the optimal gastric cancer screening method compared with no screening for employees in Japan.
Methods
Decision trees combined with Markov models were developed and constructed for two strategies: HPA screening and no screening (Fig. 1):
1. No screening
2. HPA screening: The employee undergoes HPA screening. If the HPA is positive and the employee receives H. pylori eradication treatment and the eradication treatment is effective, the risk of subsequent gastric cancer decreases. If the HPA is positive and the employee receives H. pylori eradication treatment and the eradication treatment is not effective, the risk of subsequent gastric cancer does not decrease. If the HPA is positive and the employee does not receive H. pylori eradication treatment, the risk of subsequent gastric cancer does not decrease. If the HPA is negative, the employee has no eradication treatment. Hospital approach rate for treatment, efficacy of H. pylori eradication treatment, gastric cancer rate with H. pylori infection, gastric cancer rate after successful eradication and gastric cancer rate without H. pylori infection are considered in the models.
Decision-analytical calculations were performed using Tree Age Pro 2012 (TreeAge Software Inc., Williamstown, MA, USA).
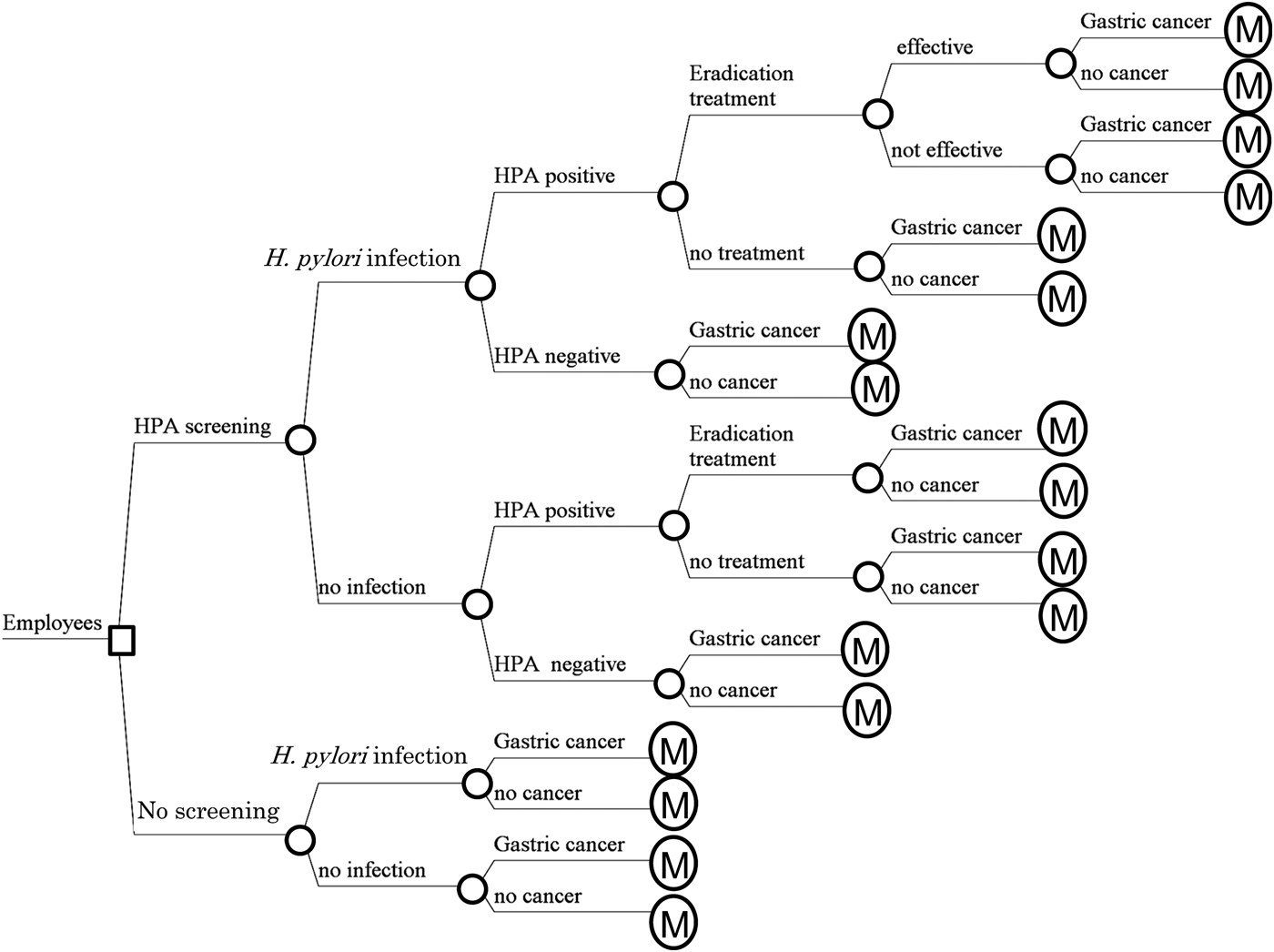
Fig. 1. Simplified decision trees. HPA, Helicobacter pylori antibody test; H. pylori, Helicobacter pylori. A square node represents the decision node. A circle node represents a chance node. Branches from a chance node represent possible outcomes. A ![]() node represents a Markov node.
node represents a Markov node.
As this was a modelling study with all inputs and parameters derived from published literature, ethics approval was not required.
Target population
Targeted populations were hypothetical cohorts of employees in high-risk populations aged 20, 30, 40, 50 and 60 years, using a company health payer perspective on a lifetime horizon.
Markov models
The following four clinical states were included in this model to represent the possible clinical states in the target populations: (i) no H. pylori infection; (ii) H. pylori infection; (iii) gastric cancer; (iv) dead (Fig. 2) [Reference Areia17]. Each cycle length was 1 year.

Fig. 2. Cohort simulation in a state-transition Markov model. H. pylori, Helicobacter pylori.
Costs, probabilities, effectiveness, utilities and other assumptions
All data were collected using MEDLINE to estimate input parameters for the model. A search of the literature published from 1980 to 23 June 2018 was undertaken to use the cost-effectiveness analysis.
All costs were adjusted to 2018 Japanese yen, using the medical care component of the Japanese consumer price index and were converted to US dollars, using the Organisation for Economic Co-operation and Development (OECD) purchasing power parity rate in 2018 (US$1 = ¥108.8). The costs of HPA screening, H. pylori eradication treatment, endoscopic screening with urea breath test and gastric cancer treatment were determined from national fee schedule in Japan [18] (Table 1).
Table 1. Baseline estimates for selected variables
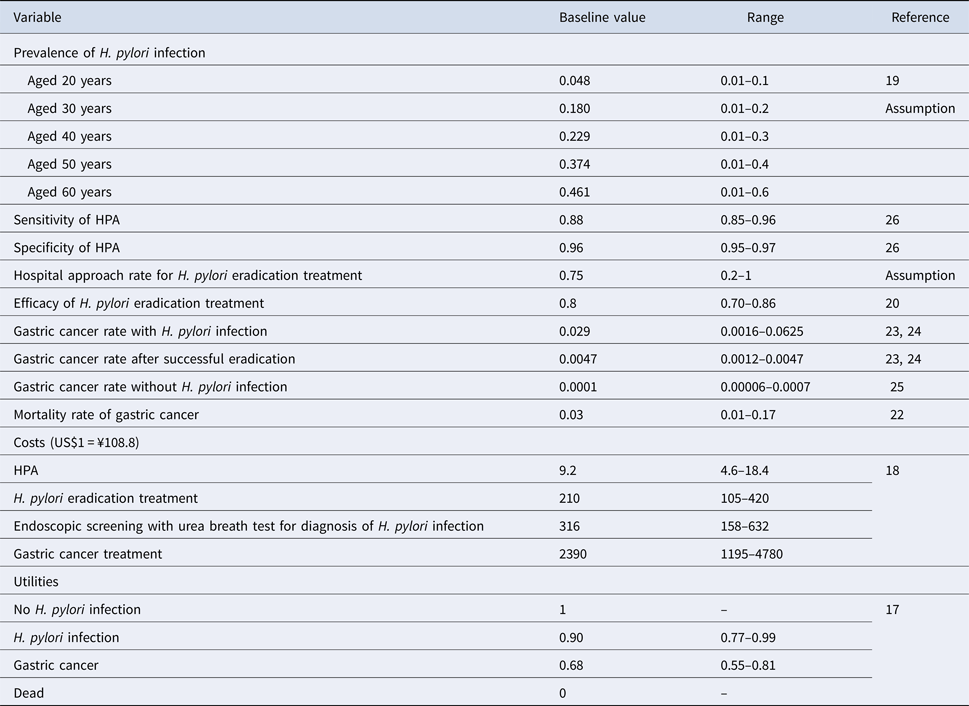
HPA, H. pylori antibody test; H. pylori, Helicobacter pylori.
Prevalence of H. pylori among employees aged 20, 30, 40, 50 and 60 years, efficacy of H. pylori infection eradication by treatment, gastric cancer rate with H. pylori infection, gastric cancer rate after successful eradication, gastric cancer rate without H. pylori infection and the mortality rate of gastric cancer were derived from published literatures [Reference Hirayama19–Reference Matsuo25]. The mortality rate due to the other causes was derived from life tables. The sensitivity and specificity of HPA were assumed from the published literature [Reference Shady26]. The hospital approach rate of H. pylori eradication treatment was assumed.
The main outcome measure of effectiveness was quality-adjusted life-years (QALYs). The use of QALYs allows us to combine the effects of quantity of life with health-related quality of life in a single measure. A QALY is a year of life lived in perfect health and 0 QALY is death. Health state utilities were obtained from the literatures and were calculated by using a utility weight (Table 1) [Reference Areia17].
Per-person costs and effectiveness were calculated and compared. Incremental cost-effectiveness ratios (ICERs) were calculated by using incremental costs and incremental QALYs gained and were compared with a willingness-to-pay level of US$100 000/QALY gained.
All costs and all clinical benefits were discounted at a fixed annual rate of 3%.
Sensitivity analyses
One-way sensitivity analyses were performed to determine which strategy was more cost-effective, using the wide ranges of probabilities, costs and utilities. The assumed ranges of one-way sensitivity analyses are shown in Table 1.
Probabilistic sensitivity analyses using the Monte-Carlo simulation were performed to assess the impact of the uncertainty in the model on the base-case estimates and recalculated expected values during 10 000 reiterations. The uncertainties in probabilities, the sensitivity and specificity of HPA were assumed to have a β distribution.
Results
In the base-case analysis, HPA screening yielded greater benefits at lower cost than no screening (20 year-old employees: HPA screening; US$90.95, 27.88409 QALYs; No screening; US$109.23, 27.79560 QALYs; 30 year-old employees: HPA screening; US$256.62, 25.80450 QALYs; No screening; US$363.73, 25.49955 QALYs; 40 year-old employees: HPA screening; US$299.56, 23.29758 QALYs; No screening; US$419.41, 22.95223 QALYs; 50 year-old employees: HPA screening; US$432.57, 20.06278 QALYs; No screening; US$595.53, 19.58230 QALYs; 60 year-old employees: HPA screening; US$467.21, 16.33820 QALYs; No screening; US$606.08, 15.86375 QALYs) (year 2018 values) (Table 2). On the one-way sensitivity analyses, cost-effectiveness was not sensitive to any variables. According to the Monte-Carlo simulations for 10 000 trials, HPA screening was more cost-effective with a value of 100% at a willingness-to-pay level of US$100 000/QALY compared with no screening (Fig. 3). The results were strongly robust.

Fig. 3. Cost-effectiveness acceptability curves. HPA, Helicobacter pylori antibody test.
Table 2. Results of base-case analyses

HPA, H elicobacter pylori antibody test; QALYs, quality-adjusted life-years; ICER, incremental cost-effectiveness ratio.
Discussion
This study demonstrated that HPA screening yielded greater benefits at lower cost than no screening among employees in Japan. One-way and probabilistic sensitivity analyses showed strong robustness of the cost-effectiveness results. The high H. pylori prevalence of employees, high gastric cancer rate with H. pylori infection, high hospital approach rate for eradication treatment and high reduction rate of gastric cancer after successful eradication may be the main reasons of higher cost-effectiveness results of HPA screening.
To the best of our knowledge, this study is the first cost-effectiveness analysis of H. pylori screening with eradication treatment for a high-risk population in the occupational health setting, using Markov models. Markov models not only have stochastic processes with transitions from one state to another state, but also allows for the simulation of more complex consequence of chronic diseases such as gastric cancer with a greater number of possible events during lengthier periods [Reference Ademi27].
Japan has the third highest age-standardised incidence of gastric cancer [2]. Prevalence of H. pylori infection by birth-year has remarkable declining trend with decreasing gastric cancer incidence and mortality [Reference Watanabe28]. Currently, gastric cancer screenings using upper gastrointestinal series and endoscopy are recommended in population-based (employee-based and community-based) and opportunistic cancer screening in Japan [Reference Hamashima3, Reference Hamashima5]. However, the screening rates are still very low. We consider which gastric cancer screening should be conducted as a policy control measure to save the lives among limited resources. To promote the effective prevention of cancer and save more lives of employees, a company can improve the screening rate and construct the new cancer screening methods on the basis of cost-effectiveness.
This study was based on the prevalence of H. pylori infection of a large-scale epidemiological study with subjects in Japan [Reference Hirayama19]. The results of this study also reflect the present cost-effectiveness of population-based H. pylori screening in Japan.
There are several cost-effectiveness studies of H. pylori screening to prevent gastric cancer. Fendrick et al. demonstrated that population-based H. pylori screening has the potential to produce important health benefits at a reasonable cost at moderate rates of excess risk reduction of cancer [Reference Fendrick29]. Xie et al. showed that the population-based serology screening for H. pylori serology screening with eradication therapy was more cost-effective than the urea breath test with eradication therapy in the prevention of gastric cancer among Chinese males in Singapore [Reference Xie, Luo and Lee30]. Roderick et al. demonstrated that once-only screening at age 40 with an initial prevalent round of those aged 40–49 is cost-effective and appears to be the most pragmatic policy in the UK. They concluded that screening at younger ages could prevent more deaths but is likely to have lower compliance [Reference Roderick31, Reference Roderick32]. Teng et al. showed that H. pylori serology-based screening was likely to be cost-effective in New Zealand, particularly for the indigenous population [Reference Teng33]. Leivo et al. demonstrated that population-based H. pylori screening is more favourable in the older age cohorts compared with no-screening in Finland [Reference Leivo34]. This study demonstrated that employee-based H. pylori screening is more cost-effective than no screening in the occupational health setting in Japan.
The current study has a number of limitations. First, there is no cohort study of HPA screening among employees. Further studies for clinical trials and well-controlled prospective studies for employees are needed. Second, the only serological screening method to calculate is the HPA in this study. There are the other H. pylori diagnostic methods: stool H. pylori antigen, bacterial culture, urine H. pylori antibody, rapid urease test and urea breath test. Third, the benefits of peptic ulcer and MALT lymphoma after eradication are not considered in this study. Fourth, reinfection and recurrence of H. pylori are not considered in this model. H. pylori infection is mainly acquired in childhood and recurrence of H. pylori infection after successful eradication is uncommon in developed countries [Reference Bruce and Maaroos35, Reference Gisbert36]. Sheu et al. found that the presence of dental disease could predispose to H. pylori recurrence [Reference Sheu37]. Fifth, the risk of other pathologies including inflammatory bowel diseases, oesophageal adenocarcinoma, metabolic syndrome and asthma after H. pylori eradication is not considered in this model. Further cost-effectiveness studies including the association with those diseases will be needed [Reference Xiang38–Reference Refaeli42]. Finally, there are different costs and medical systems in each country. The costs, H. pylori prevalence and gastric cancer risk of Japanese were used in this study. Further cost-effectiveness studies by the variance of each country will be needed.
In conclusion, H. pylori screening followed by eradication treatment is recommended to prevent gastric cancer for employees in Japan, on the basis of the benefits and cost-effectiveness.
Acknowledgements
No funding was received for this study.
Conflict of interest
The author reports no conflicts of interest that are directly relevant to the content of this study. The author has no financial disclosures.








