Introduction
In December 2019, a cluster of pneumonia cases of unknown cause occurred in Wuhan city of China [Reference Wang1]. In early January 2020, a novel betacoronavirus was isolated [Reference Zhu2] from the bronchoalveolar lavage samples of the infected patients, and the pathogen was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously known as 2019 novel coronavirus, 2019-nCoV). In February 2020, the WHO officially designated the syndrome as coronavirus disease 2019 (COVID-19).
Due to human-to-human transmission [Reference Chan3, Reference Li4], COVID-19 has spread rapidly. As of 27 March 2020, a total of 82 078 cases have been confirmed in China and 509 164 cases have been reported in more than 200 countries and five continents [5]. The clinical spectrum of COVID-19 appears to be wide, including asymptomatic infection, mild respiratory tract illness and severe pneumonia with respiratory failure and even death [Reference Singhal6]. The mortality of COVID-19 is different among countries and regions, for instance 4.02% in China, 0% in Vietnam, 10.14% in Italy, 1.45% in USA, and 0.44% in Australia [5].
So far, studies on the epidemic and clinical characteristics of COVID-19 have mainly concentrated on initial or first-generation cases. Information about imported and second-generation cases is limited. In this study, we focused on Shaanxi province as a region with imported and second-generation cases and described the clinical and laboratory characteristics of 134 COVID-19 cases in this province with a hope to provide some insight into the prevention and treatment of the disease in China and elsewhere.
Methods
Study design and participants
This retrospective study included 134 confirmed cases of COVID-19 admitted and treated in 10 designated hospitals across nine cities (Xi'an, Ankang, Baoji, Hanzhong, Weinan, Xianyang, Shangluo, Yan'an and Tongchuan) in Shaanxi province from 23 January 2020 to 7 March 2020 (Supplementary Table S1). SARS-CoV-2 infection was defined in accordance with Version 7.0 of the guideline issued by the National Health Commission of the People's Republic of China [7].
Data collection
The epidemiological, demographic, clinical, laboratory and radiologic characteristics as well as treatment and outcome data were collected from patients' electronic medical records using a standardised case report form. Clinical outcomes were followed up until 7 March 2020. The data were reviewed by a trained team of physicians. If information was not clear, the research team contacted the doctor responsible for treating the patient for clarification.
Laboratory confirmation
Laboratory confirmation of COVID-19 was performed immediately after admission and verified by the Shaanxi Provincial Centre for Disease Control and Prevention (CDC). A confirmed COVID-19 case was defined as a positive result to high-throughput sequencing or real-time reverse-transcriptase polymerase chain reaction assay for nasal and pharyngeal swab samples or sputum specimens [8].
Diagnostic criteria
The date of disease onset was defined as the day when the symptom was noticed. Fever was defined as axillary temperature above 37.3 °C. ARDS was defined in accordance with the Berlin definition [Reference Force9]. Acute kidney injury was identified based on the Kidney Disease: Improving Global Outcomes definition [Reference Khwaja10]. Cardiac injury was determined when the serum levels of cardiac biomarkers (e.g. troponin I/T) were above the 99th percentile upper reference limit or new abnormalities detected in electrocardiography and echocardiography [Reference Gao11]. Ventilator-associated pneumonia was determined referring to the guidelines for treatment of hospital-acquired and ventilator-associated pneumonia [Reference Kalil12]. Severity of COVID-19 was categorised into non-severe group (mild and moderate) and severe group (severe and critically ill) based on Version 7.0 of the guideline issued by the National Health Commission of the People's Republic of China [7].
Statistical analysis
The cohort of patients was divided into severe and non-severe cases. Continuous and categorical variables were expressed as median (interquartile range (IQR)) and n (%), respectively. The Mann–Whitney test was used for continuous variables and χ 2 test or Fisher's exact test (when the data were limited) was used for categorical variables to compare differences between severe and non-severe cases where appropriate. All statistical analyses were performed with SPSS software, version 23.0. A two-sided α of less than 0.05 was considered statistically significant.
Result
Epidemiological and clinical characteristics
This study recruited a total of 134 confirmed COVID-19 patients from nine cities in Shaanxi province from 23 January 2020 to 7 March 2020. The median age of the patients was 46 years old (IQR 34–58), ranging from 4 to 89 years, and more than half (69, 51.5%) were female (Table 1). Altogether 88 (65.7%) cases were non-severe and 46 (34.3%) were severe, including two critically ill cases (1.5%) with one patient unable to survive (0.7%) (Table 4). The ages of severe patients were significantly older than that of non-severe patients (median, 56 years vs. 41 years, P < 0.05). Moreover, the proportion of patients aged 65 or older was higher (32.6% vs. 5.7%, P < 0.05), and the proportion of patients aged 14–30 was lower (4.3% vs. 21.6%, P < 0.05) in the group of severe patients than in non-severe patients (Table 1).
Table 1. Demographics, baseline characteristics of patients infected with COVID-19
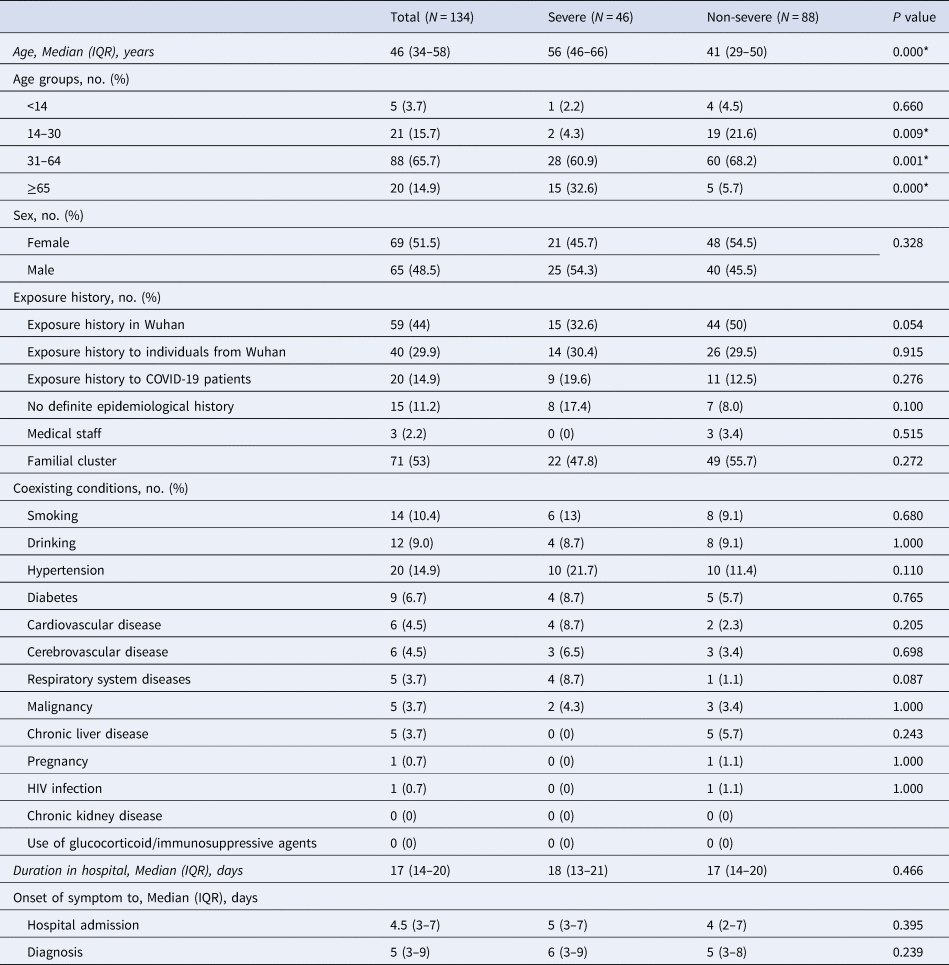
COVID-19, coronavirus disease-19; IQR, interquartile range.
P values indicate differences between severe and non-severe patients. P < 0.05 was considered statistically significant.
None of the patients had a history of exposure to the Huanan Seafood Market or wild animals. The majority of the cases were community-infected and three cases were hospital-infected. Of these patients, 59 (44%) resided in Wuhan or had short trips to Wuhan before the onset of COVID-19; 40 (29.9%) had close contact with someone from Wuhan; 20 (14.9%), including three (2.2%) medical staff, had exposure to COVID-19 patients; 15 (11.2%) had no definite epidemiological history and 71 (53%) patients got infected as familial clustering (Table 1).
Of the 134 patients, 58 (43.3%) had one or more coexisting medical conditions, the most common of which was hypertension (14.9%), followed by diabetes (6.7%), cardiovascular disease (4.5%) and cerebrovascular disease (4.5%) (Table 1). The most common symptoms at onset were cough (96, 71.6%), followed by fever (87, 64.9%) (Table 2). The incidences of chest stuffiness and dyspnoea differed between severe and no-severe cases (Table 2, both P < 0.05). The median interval from onset of symptoms to first hospital admission was 4.5 (IQR 3–7) days, and that to positive result of nucleic acid detection was 5 (IQR 3–9) days. The median duration from hospital admission to discharge was 17 days (IQR 14–20) (Table 1).
Table 2. Symptomatic and radiological characteristics of patients infected with COVID-19
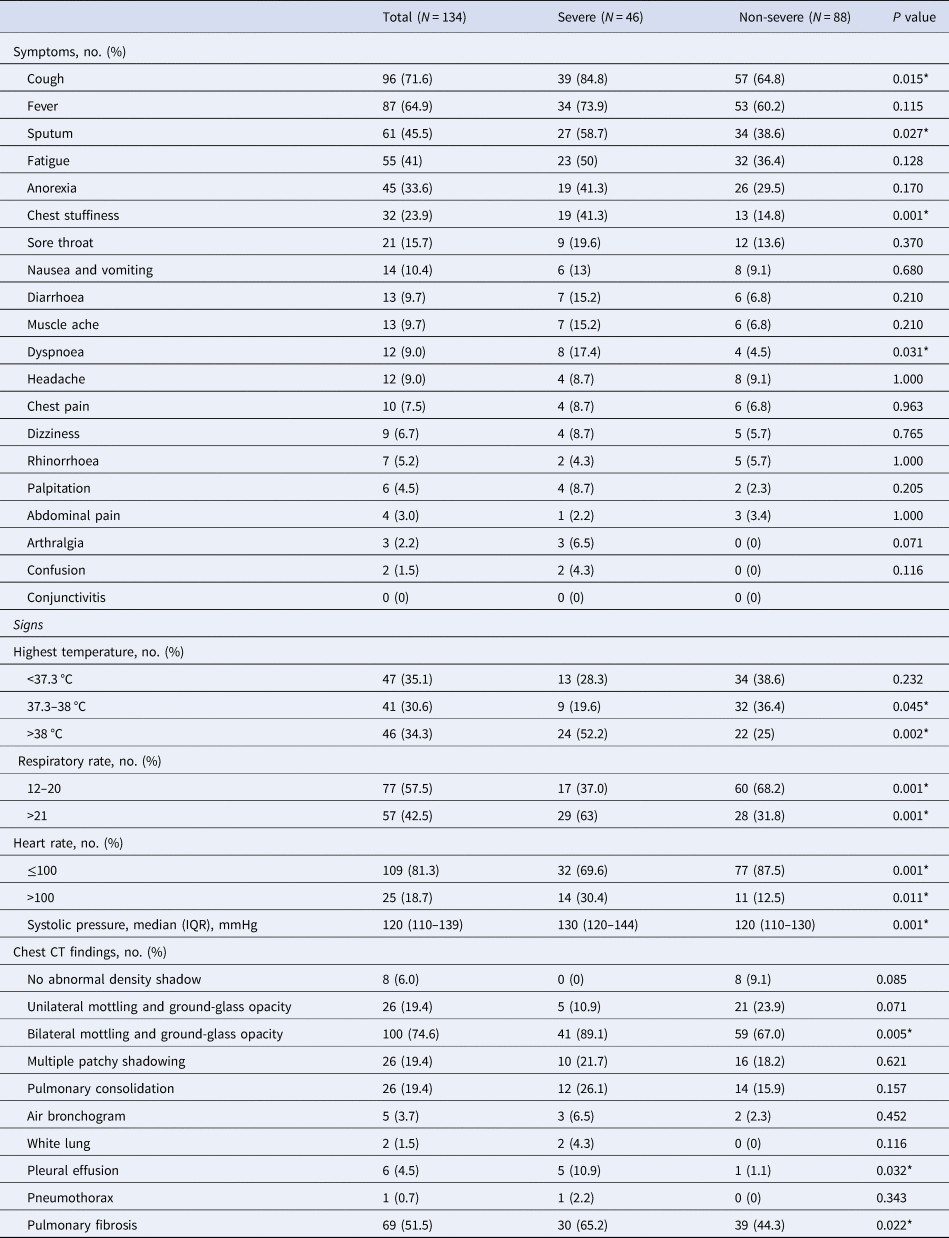
P values indicate differences between severe and non-severe patients. P < 0.05 was considered statistically significant.
The incidences of temperature >38 °C, respiratory rate >21 breaths per min, heart rate >100 beats per min and median systolic pressure showed difference between severe and no-severe cases (Table 2, all P < 0.05). All of these were higher in severe cases compared to in non-severe cases.
Radiological and laboratory findings
Ninety-four per cent (126/134) of the patients showed abnormal chest computed tomography (CT) images, consisting of 26 cases (26/134, 19.4%) of unilateral pneumonia and 100 cases (100/134, 74.6%) of bilateral pneumonia, with ground-glass opacity as the typical hallmark finding. Among the patients, 26 patients (19.4%) showed multiple patchy shadowing, 26 cases (19.4%) showed subsegmental consolidation with air bronchogram (5, 3.7%), with two cases (1.5%) having progressed to ‘white lung’. Additionally, pleural effusion occurred in six patients (4.5%) and pneumothorax occurred in one patient (0.7%). When the shadow or consolidation was resolved, pulmonary fibrosis was found on later chest CT images of 69 (51.5%) patients (Table 2). Moreover, the incidences of bilateral pneumonia, pleural effusion and pulmonary fibrosis were higher in severe cases than in non-severe cases (Table 2, all P < 0.05).
Upon admission, 25 (18.7%) of the patients showed leucopoenia (white blood cell count <3.5 × 109/l) and 51 (38.1%) showed lymphopoenia (lymphocyte count <1.1 × 109/l). In most patients, leucocytes (107, 79.9%) and lymphocytes (82, 61.2%) were within the normal ranges. The median values of C-reactive protein (CRP) (10.0, IQR 9.0–38.3 mg/l), erythrocyte sedimentation rate (ESR) (38.5, IQR 17.8–74.8 mm/h), interleukin-6 (IL-6) (8.1, IQR 5.0–23.0 pg/ml) and direct bilirubin (DBIL) (4.9, IQR 3.3–7.5 μmol/l) elevated in the patients. The median partial pressure of oxygen level was 80 mmHg (IQR 67–92), and the median of oxygenation index (PaO2:FiO2) was 255 mmHg (IQR 210–307) (Table 3).
Table 3. Laboratory findings of patients infected with COVID-19
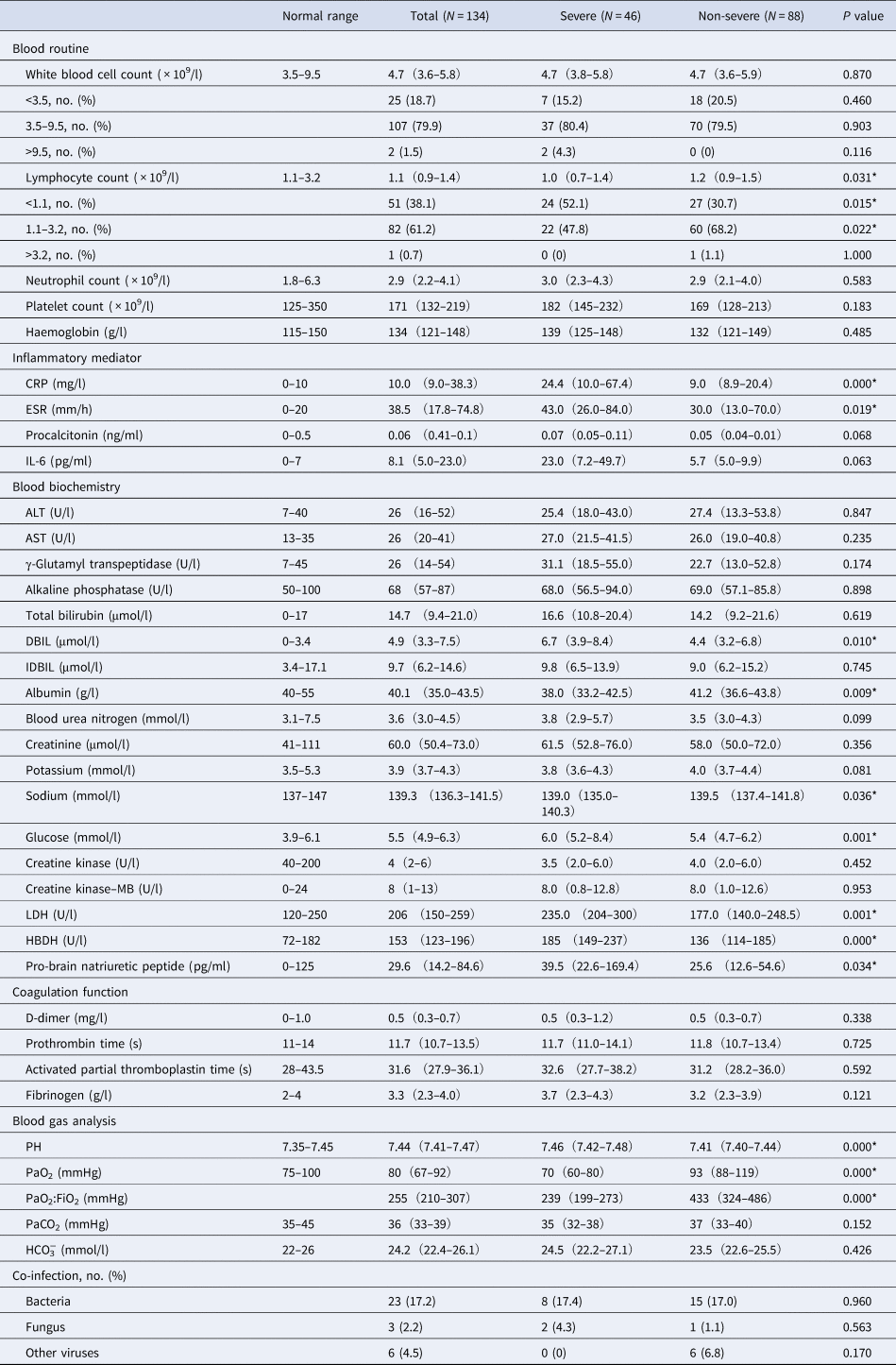
Values are medians (interquartile ranges) unless stated otherwise.
PaO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen; PaCO2, partial pressure of carbon dioxide.
P values indicate differences between severe and non-severe patients. P < 0.05 was considered statistically significant.
A number of laboratory parameters showed higher values in severe patients as compared with non-severe patients (Table 3), including CRP, ESR, DBIL, glucose, lactate dehydrogenase (LDH), hydroxybutyrate dehydrogenase (HBDH) and pro-brain natriuretic peptide (Table 3, all P < 0.05). In addition, lymphocyte count, albumin, PaO2 and PaO2:FiO2 were comparatively lower in severe patients than in non-severe patients (Table 3, all P < 0.05).
Complications, treatment and outcomes
During hospitalisation, 15 (11.2%) of the patients had complications, including arrhythmia (4, 3.0%), acute respiratory distress syndrome (3, 2.2%), acute kidney injury (3, 2.2%), ventilator-associated pneumonia (2, 1.5%), multiple organ dysfunction syndrome (2, 1.5%) and shock (1, 0.7%). Most of the complications (13 out of 15, 86.7%) occurred in the group of severe cases and the incidence of complications was comparatively higher in severe cases than in non-severe cases (28.3% vs. 2.3%, P < 0.05) (Table 4).
Table 4. Complications, treatment and outcomes of patients infected with COVID-19

P values indicate differences between severe and non-severe patients. P < 0.05 was considered statistically significant.
As for therapeutic management, 91 (67.9%) patients received oxygen inhalation, the two critical illness cases (1.5%) were treated with noninvasive ventilation, of whom one switched to invasive mechanical ventilation (IMV), extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT) as salvage therapy, and another one died before switching to IMV (Table 4).
Of the 134 patients, 23 (17.2%) experienced a secondary bacterial infection, three (2.2%) were detected as positive for secondary fungus infection and six (4.5%) had other viruses infection (Table 3). Empirical single antibiotic treatment, mainly moxifloxacin, was given to 103 patients (76.9%), with a median duration of 10 days (IQR 7–14). Most patients (129, 96.3%) received antiviral therapy (median duration 13 days, IQR 8–17), including lopinavir/ritonavir (87, 64.9%), interferon alpha inhalation (68, 50.7%), arbidol (57,42.5%), ribaviron (44, 32.8%) and chloroquine (3, 2.2%). The median interval from onset of symptoms to antiviral therapy was 6.0 (IQR 4–9) days (Table 4). Additionally, two patients (1.5%) received antifungal treatment (Table 4).
Glucocorticoid therapy (median duration 3 days, IQR 2.0–5.5) was performed in 41 patients (30.6%), the duration of which was remarkably longer in severe cases than in non-severe cases (median 5 vs. 3, P < 0.05). The median interval from onset of symptoms to glucocorticoid therapy was 6 days (IQR 5.0–10.3). In addition, 13 cases (9.7%) were supported with gamma globulin (median 4 days, IQR 3.0–7.0). Significantly more severe cases were given oxygen inhalation, antibiotics, systematic corticosteroid and gamma globulin (all P < 0.05, Table 4).
By 7 March 2020, 123 (91.8%) of the 134 patients had been discharged and one critical patient (0.7%) had died. The remaining ten patients (7.5%) still under treatment were largely severe cases (8 out of 46 severe, 17.4% vs. 2 out of 88 non-severe, 2.3%, P < 0.05) (Table 4). Fitness for discharge was based on abatement of fever for at least 3 days, significantly improved respiratory symptoms, and negative result for two consecutive respiratory pathogenic nucleic acid tests (sampling interval at least 1 day).
Dynamic profile of laboratory findings in patients with COVID-19
To determine the major clinical features during COVID-19 progression, the dynamic changes of six clinical laboratory parameters, namely, lymphocyte, IL-6, CRP, LDH, HBDH and DBIL, were monitored every other day from day 1 to day 8 after hospital admission. By 7 March 2020, data of the complete clinical course from seven patients, including five randomly selected discharged patients, two critically ill cases (one managed with ECMO and one died) were analysed (Fig. 1). Baseline lymphocyte count was significantly higher in survivors than in the non-survivor patient. In survivors, the lymphocyte count was lowest on day 5 after admission and increased gradually during hospitalisation, whereas, the non-survivor patient developed more severe lymphopoenia (0.19 × 109/l) over time. The level of IL-6 in survivors displayed a gradual decrease to normal range with the condition improved, but increased unexpectedly to a very high value (5001 pg/ml) before death in the non-survivor case.
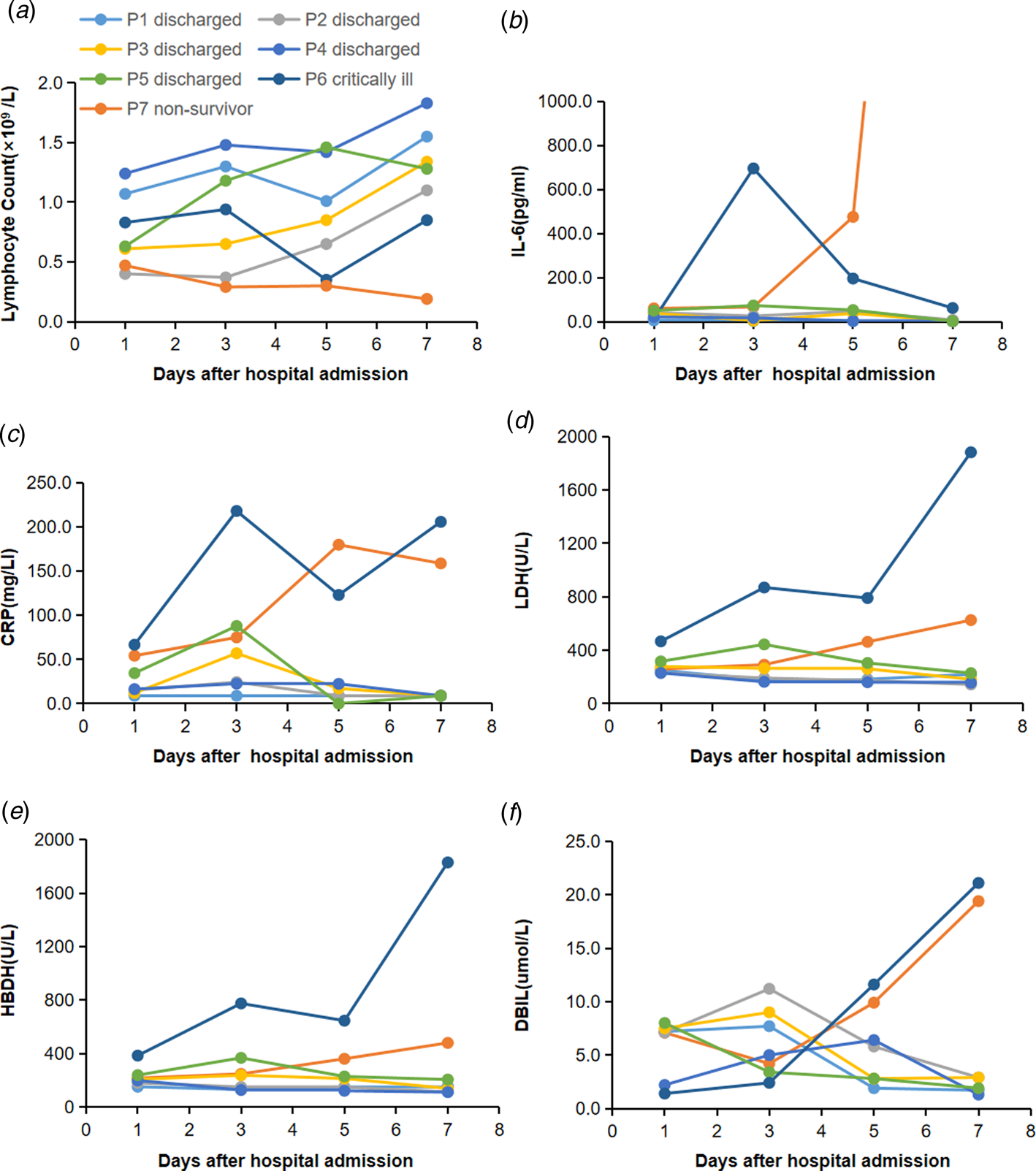
Fig. 1. Dynamic profile of laboratory findings in patients with COVID-19. Timeline charts illustrated the dynamic changes of six laboratory markers (lymphocyte, IL-6, CRP, LDH, HBDH and DBIL) in seven COVID-19 patients (five discharged cases, two critically ill cases including one managed with ECMO and one non-survivor) every other day after hospital admission. (a–f) Dynamic changes of lymphocyte (a), IL-6 (b), CRP (c), LDH (d), HBDH (e), DBIL (f). The descriptive curve of individual patient: discharged/cured cases: P1, P2, P3, P4, P5; critically ill cases: P6; non-survivor: P7 was displayed. IL-6, interleukin-6; CRP, C-reactive protein; LDH, lactate dehydrogenase; HBDH, hydroxybutyrate dehydrogenase; DBIL, direct bilirubin; COVID-19, coronavirus disease-19.
Compared with those in the recovered patients, levels of CRP, LDH, HBDH and DBIL in the two critically ill patients were higher throughout the clinical course (Fig. 1). In the recovered patients, the levels of all the four markers reached the peak on day 3 after admission and decreased subsequently during recovery. In the two critically ill cases, the levels increased rapidly from day 3 with condition deterioration.
Discussion
Fifty-six per cent of the patients enrolled in this multicentre study had never been to Wuhan and had been infected outside Wuhan. This suggests a gradual shift of initial infection to second-generation local infection which should be taken into account.
The percentage of male patients in our data was 48.5%, different from the male patient predominance reported in two studies on Wuhan cases (73% in Huang et al. [Reference Huang13] and 68% in Chen et al. [Reference Chen14]). In this study, the male–female ratio was approximately 1:1.06, with no difference between severe and non-severe cases. This finding is contradicting to the previous conclusion that men were more susceptible than women to SARS-CoV-2 [Reference Chen14, Reference Chen15]. This might be related to occupational exposures, for more men than women work as salesmen or market managers in seafood markets. As recorded, 66.0% of the patients in Huang's report and 49% of the patients in Chen's report had the history of exposure to the Huanan Seafood Market, and most of the affected patients were male workers [Reference Huang13, Reference Chen14]. In contrast, no patient in our study had such exposure. All of these indicated a change of transmission mode outside Wuhan and that gender may not be a susceptible factor for COVID-19.
The median age of our patients was 46 years old, close to that of patients outside Wuhan as reported by Wu et al. (46 years) [Reference Wu16] and Xu et al. (41 years) [Reference Xu17], and younger than that of patients in Wuhan as reported by Wang et al. (56 years) [Reference Wang18] and Chen et al. (55 years) [Reference Chen14]. Similarly, severe patients were much older than non-severe patients. This suggests that age may be an important risk factor for poor outcome. The role of age in COVID-19 seems to be similar to its role in SARS and MERS, which has been reported as an independent predictor of adverse outcome [Reference KW19, Reference Hong20]. T-cell and B-cell hypofunction and excessive production of type 2 cytokines in older people could lead to defect in inhibition of viral replication and stronger host innate responses with sustained cytokine storm, potentially leading to poor outcome [Reference Opal, Girard and Ely21]. Therefore, compromised immune function might be the major cause of higher mortality in older people infected by coronaviruses.
The proportion of severe cases in Shaanxi was close to that in Wuhan as reported previously [Reference Huang13, Reference Chen14, Reference Zhang22], while the incidences of complications and mortality were considerably lower among Shaanxi patients than among the initially infected Wuhan patients [Reference Huang13, Reference Chen14, Reference Zhang22] (Supplementary Table S2, both P < 0.05). Only two cases in our cohort needed mechanical ventilation, the incidence of which was much lower than that reported in Wuhan cases [Reference Huang13, Reference Chen14, Reference Zhang22] (Supplementary Table S2, P < 0.05). This might indicate that patients outside Wuhan had a much better prognosis than the first generation patients in Wuhan. What's more, of the cases in Wuhan, those initially identified had a higher mortality than those confirmed and treated later (15% [Reference Huang13] vs. 11% [Reference Chen14] vs. 4.3% [Reference Wang18]). This phenomenon was similar to that during the transmission of MERS-CoV, in which the global mortality of the first-generation MERS-CoV was about 35.5%, while that of the second-generation was around 20% [Reference Christl23]. Furthermore, the median interval from symptom onset to hospital admission in Shaanxi cases was shorter than in Wuhan cases (4.5 vs. 7 days) [Reference Huang13, Reference Wang18] and the Shaanxi patients were younger than those in Wuhan (46 vs. 55–62 years) [Reference Chen14, Reference Chen15, Reference Wang18]. These may be reasons for the notable reduction in mortality in Shaanxi cases.
The percentage of cases having fever in our cohort was lower than that reported in Wuhan [Reference Huang13, Reference Chen14, Reference Wang18]. In this regard, patients with normal temperature may be missed if the surveillance case definition focused heavily on fever detection. Compared with non-severe patients, severe patients more commonly had symptoms and signs such as cough, sputum, chest stuffiness, dyspnoea, temperature above 38 °C, respiratory rate above 21 breaths per min, and heart rate above 100 beats per min. The onset of symptoms and signs may assist physicians in identifying patients with greater severity.
Based on the radiological data, the incidences of bilateral pneumonia and pleural effusion were higher in severe cases than in non-severe cases, which suggested greater disease severity. Similar to what was reported by Sun et al. [Reference Sun24], in 54.7% (69/126) of the pneumonia cases, pulmonary fibrosis was found in later chest CT images when shadowing had been resolved, and the phenomenon was more common in severe patients than in non-severe patients. These findings consistently suggest that pulmonary fibrosis can be one of the sequelae of COVID-19. It is necessary and important to explore how to prevent and reduce the occurrence of pulmonary fibrosis and how to manage the situation whenever it occurs in the treatment of COVID-19.
In terms of laboratory tests, different from cases in Wuhan, most Shaanxi patients had lymphocytes within the normal range, and only 38.1% showed lymphopoenia. The lymphocyte absolute count in our cohort of patients (1.1 × 109/l) was higher than that reported in Wuhan patients (0.6–0.8 × 109/l) [Reference Huang13, Reference Wang18, Reference Zhou25]. This may be another reason for the lower mortality of Shaanxi cases as compared with of Wuhan cases. In severe cases, the lymphocyte count was lower and the incidence of lymphopoenia was higher than in non-severe cases. These findings suggest that SARS-CoV-2 might mainly act on lymphocytes, especially T lymphocytes, and the severity of lymphopoenia might reflect the severity of the disease. Furthermore, levels of inflammatory parameters, such as CRP and ESR elevated in COVID-19 patients and were even higher in severe patients. The changes in these laboratory parameters illustrated that the virus invaded through respiratory mucosa and spread in the body, triggering a series of immune responses and inducing severe inflammation and cytokine storm in vivo [Reference Felsenstein26].
Few patients in our study had abnormal levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and indirect bilirubin (IDBIL). The median level of DBIL in the patients elevated, and was even higher in severe patients. As reported, the potential mechanism of liver dysfunction in COVID-19 could be that the virus might directly bind to ACE2 positive bile duct cells [Reference Wan27]. Therefore, the liver abnormality of COVID-19 patients may not be caused by liver cell damage, but by bile duct cell dysfunction.
In addition, elevated glucose, LDH, HBDH and pro-brain natriuretic peptide, as well as declined albumin, PaO2 and PaO2:FiO2, were more commonly seen in severe cases, suggesting greater disease severity.
The dynamic changes of six laboratory markers showed that baseline lymphocyte count was significantly higher in survivors than in the non-survivor patient, and it increased as the condition improved, but declined sharply when death occurred. Conversely, the IL-6 level displayed a downtrend in survivors, but continually rose to a very high level in the non-survivor patient. Hence, we assume that T cellular immune function might relate to mortality, and lymphocyte and IL-6 should be used as indicators for prognosis. Additionally, CRP, LDH, HBDH and DBIL levels decreased as the condition improved in recovered patients, but increased rapidly as the condition worsened in the two critically ill cases. These may be related to cytokine storm and bile duct cell dysfunction induced by virus invasion.
Most patients (96.3%) in our study received antiviral therapy, including lopinavir/ritonavir (64.9%), interferon alpha inhalation (50.7%), arbidol (42.5%), ribaviron (32.8%) and chloroquine (2%). Until now, no specific treatment has been recommended for COVID-19 infection except for optimal supportive care. Currently, randomised clinical trials for lopinavir/ritonavir (ChiCTR2000029308) and intravenous remdesivir (NCT04257656, NCT04252664) in treatment of COVID-19 showed no benefit with lopinavir/ritonavir [Reference Cao28] or remdesivir treatment [Reference Wang29] beyond standard care. Meanwhile, COVID-19 vaccine is highly expected. Ongoing efforts are needed to explore effective therapies for this emerging acute respiratory infection.
Limitations of the study
This study has some limitations. First, as only COVID patients in Shaanxi were recruited, our conclusions need to be further verified by recruiting larger number of cases of other provinces or cities, outside Wuhan. Second, there were two critically ill cases with one non-survivor in our study, thus dynamic observations of laboratory parameters between non-survivor and survivor, recovered cases and critically ill cases were just descriptive analysis. Larger number of critically ill cases are needed to verify our observation.
Conclusion
In summary, the present study identified that the imported and second-generation COVID-19 cases in Shaanxi had a better prognosis in comparison with initial or first-generation cases in Wuhan, with less complications and lower fatality. These differences may be related to the shorter interval from symptom onset to hospital admission, younger age and higher lymphocyte count of patients in Shaanxi. Lymphocyte count and IL-6 level could be used as indicators for evaluating prognosis. CRP, LDH, HBDH and DBIL levels could help estimate the severity and development tendency of the disease. Pulmonary fibrosis was found in later chest CT images in more than half of the pneumonia cases and should be taken into account.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268820002332
Acknowledgments
We are grateful to all health-care workers involved in the diagnosis and treatment of COVID patients. We thank all our colleagues who helped us during the current study.
Author contributions
Conception and design: PYS, GXR, JY, MWC; Administrative support: ZFX, ZXW; Provision of study materials or patients: PYS, GXR, JY, ZQL, SJD, ML, SSW, XFX, FPC, YJL, CYL, XHY; Collection and assembly of data: PYS, ZFX, ZXW; Data analysis and interpretation: PYS, GXR, JY, MWC; Manuscript writing: all authors; Final approval of manuscript: all authors.
Financial support
This work was supported by the Major Projects of Ministry of Science and Technology of the People's Republic of China (No. 2017ZX10103004-010; to Mingwei Chen); Science and Technology Planning Project of Xi ’an (No. 20200003YX003(3); to Mingwei Chen).
Conflict of interest
The authors declare that they have no conflicts of interest.
Data availability statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.








