INTRODUCTION
Parvovirus B19, discovered in 1975, is the only parvovirus known to cause clinical disease in humans and is the sole member of the Erythrovirus genus [Reference Cohen and Mortimer1]. It replicates in the red cell precursors in the bone marrow, hence major complications can be a transient aplastic crisis and persistent anaemia. The main transmission route is through respiratory secretions, particularly in environments where close contact occurs [Reference Zaaijer, Koppelman and Farrington2]. Transmission can also occur vertically from mother to foetus, and blood products containing the virus represent a further source of infection [Reference Zaaijer, Koppelman and Farrington2]. Whilst vaccines are under development they are yet to be used routinely and parvovirus B19 shows a 3- to 5-year epidemic cycle with a seasonal peak in the first half of each year. Recent epidemic years in the United Kingdom have been 1989–1990, 1993–1994, 1997–1998 and 2002–2003.
Whilst infection with B19 is often asymptomatic, the most common clinical presentation is erythema infectiosum (also known as fifth disease and slapped cheek syndrome). This is characterized by a maculopapular rash and is clinically similar to rubella, with laboratory tests being required to reliably distinguish between the two diseases [Reference Cohen and Mortimer1, Reference Gay3]. It is also responsible for acute aplastic anaemia, which can persist in those that are immunocompromised. Infection in adults is also associated with rheumatological problems. However, the most important clinical manifestation is maternal infection during pregnancy, which can cause severe adverse outcomes such as fetal death and hydrops fetalis [Reference Cohen and Mortimer1, Reference Zaaijer, Koppelman and Farrington2]. A recent study found that the risk of fetal death following maternal infection was confined to the first 20 weeks of pregnancy and to be an average of 9%, and also a 2·9% risk of hydrops fetalis following maternal infection between 9 and 20 weeks of gestation [Reference Miller4].
Lifelong immunity is thought to result after infection with parvovirus B19 and previous antibody prevalence studies suggest the majority of adults have evidence of past infection [Reference Kelly5]. Two antibody prevalence studies across the complete age range have previously been carried out in the United Kingdom. The first used an ‘in-house’ antibody-capture radioimmunoassay to screen sera collected in 1982, 1983, 1985 and 1986 from a variety of sources [Reference Cohen and Buckley6]. A second more recent study screened a far larger number of samples [Reference Gay3] from a convenience collection of sera provided by public health laboratories in England and Wales in 1991 [Reference Osborne7] that is considered to reflect the general population [Reference Kelly8] and employed a commercial ELISA (MRL Diagnostics, Cypress, CA, USA). This assay had a poor ability to detect low concentrations of IgG and a mixture modelling technique was necessary to help interpret the serological data [Reference Gay9]. These data were also used to estimate the incidence of maternal infection with parvovirus B19 in pregnancy [Reference Gay3], suggesting that 1600–2200 mothers are infected in pregnancy in each year. From this 150–200 fetal deaths were calculated to result annually, using an estimate of 9% for the risk fetal of death following a maternal infection that was applied over the complete duration of the pregnancy [10].
In this study we again investigate antibody prevalence to parvovirus B19 in England and Wales across the complete age range. As before, sera from a convenience collection reflecting the general population were used, but this time obtained in 1996. An alternative commercial ELISA (Mikrogen; Euribel, Brussels, Belgium) was employed and data were analysed both qualitatively and quantitatively by sex and region in addition to age, and the average annual force of infection estimated in key age groups. Comparison with all available laboratory confirmation data for cases of parvovirus B19 infection in England and Wales is also included.
MATERIALS AND METHODS
Samples
A total of 2835 serum samples (collected in 1996) across the age range from persons aged 1–79 years were used. Of these, 1477 (52%) were from females and 1358 (48%) from males. All were part of a convenience collection consisting of anonymized residues of specimens submitted for microbiological or biochemical testing to 19 laboratories in England and Wales that were then part of the Public Health Laboratory Service (PHLS) and contributing to the PHLS Serological Surveillance Programme (now the HPA Seroepidemiology Programme) [Reference Osborne7].
Laboratory methods
All serological tests were performed at Lancashire Teaching Hospitals NHS Trust using the Mikrogen RecomWell Parvovirus B19 IgG ELISA (Euribel, Brussels, Belgium). Results were expressed quantitatively as a unitage (U/ml) according to the manufacturer's instructions.
Data
Individual quantitative results were aggregated into ten age groups (1–4, 5–9, 10–14, 15–19, 20–24, 25–29, 30–34, 35–39, 40–44 and ⩾45 years) by 34 reactivity categories (equal width bands based on the log10 of the calculated unitage) to enable a frequency distribution to be viewed. Samples giving a signal that lay beyond the limit of the measurement threshold of the detection system used were reported as having an optical density of ⩾3·0, and a value of 3·0 used when calculating a unitage so further quantitative analysis could be undertaken.
Fixed cut-off
Mixture models [Reference Gay9, Reference Gay11–Reference Vyse13] enable the proportion of a population with (and without) evidence of specific antibody to be estimated, but do not classify results individually according to antibody status, which is necessary for further statistical modelling at both the qualitative and quantitative level. A fixed cut-off point can be used to do this, and mixture modelling can assist with choosing an appropriate break-point to serve this function. The frequency distribution of quantitative serological data was therefore modelled as previously described, using three normal component distributions to describe the data. These reflect distributions of results considered to represent those negative, positive and strongly positive for parvovirus B19-specific IgG, displayed using equal-width reactivity groups. The total number of samples falling into each reactivity category and those estimated as having evidence of parvovirus B19-specific IgG by the mixture model can then be calculated, assuming an overall prevalence estimate in the population. A positive predictive value (PPV) for each reactivity group can subsequently be calculated by comparing the number of samples estimated to contain specific IgG in each reactivity group by the model to the total number of samples falling in this reactivity group. This estimates the proportion of those identified as testing positive that are likely to be true positives. This was carried out assuming a variety of different overall prevalence estimates for parvovirus B19 in the population screened, ranging from 40% to 70%.
Statistical methods
Samples were classified individually according to age group (as previously described), sex, region and parvovirus B19 IgG status. Region was classified as East and South East (44·4%), North (36·7%), or South West and Wales (18·9%) according to the location of the laboratory providing the sample. Parvovirus B19-specific IgG status for individual samples was assigned using a fixed cut-off that gave a PPV of 95%, using the modelled frequency distribution of quantitative results and assuming an overall prevalence of 50% in the sample population screened. A multivariable analysis of the qualitatively categorized data using logistic regression was then undertaken, the final model including a factor for age (stata 8.2; StataCorp., College Station, TX, USA).
Those with evidence of specific IgG were further analysed quantitatively using the log10 of the calculated unitage. A censored normal regression model (stata 8.2) was employed to account for those results that lay beyond the measurement threshold of the ELISA plate reader. The final model included factors for age and sex.
Force of infection
The age-specific force of infection was modelled as previously described [Reference Gay3] using a generalization of the relationship for an age-independent force of infection where the age-specific force of infection, λ(a), is related to the prevalence at age a, P (a), using the equation below.
Estimates of force of infection were therefore made from the serological data in age groups 1–4, 5–9, 10–14 and 15–44 years using a maximum-likelihood technique. Likelihood-based 95% confidence intervals for the force-of-infection estimates in each age group were obtained by finding the maximum and minimum values for which the deviance, minimized with respect to the other parameters, was within 3·84 of the minimum. Using mid-1996 population estimates and maternity estimates for England and Wales obtained in 1996 (www.statistics.gov.uk), the 95% confidence intervals around the value derived for the force of infection in those aged 15–44 years was used to estimate the range for average annual incidence of parvovirus B19 infection in females of childbearing age in each 5-year age group as previously described [Reference Gay3]. In contrast to previous estimates [Reference Gay3], clinical outcomes following maternal infection during pregnancy were calculated assuming an average 9% risk of fetal death following infection in the first 20 weeks of pregnancy and a 2·9% risk of hydrops fetalis following maternal infection between weeks 9 and 20 of gestation [Reference Miller4].
Laboratory-confirmed data
Laboratory confirmations of parvovirus B19 infection identified throughout England and Wales that were sent to the Health Protection Agency Centre for Infections (HPA CfI) were available for the years 1993–2005 with information on age and sex. Where available, information relating to pregnancy was stored in a comments field. A descriptive analysis of these data is included for comparison with the analysis of antibody prevalence data generated by this study.
RESULTS
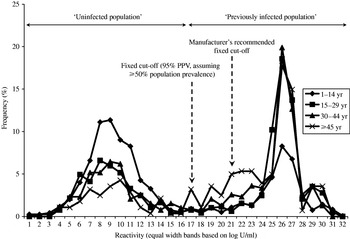
A frequency distribution of age-stratified quantitative serological data is shown by Figure 1. Three normal distributions were used to model these data, the mean (and standard deviation) that describe each being 4·5 U/ml (0·25), 151·2 U/ml (0·40) and 234·5 U/ml (0·07) respectively, and the model provided a reasonable fit to the data (deviance 522 using 294 d.f.). The component distribution with the lowest mean was particularly distinct from the other two distributions, and encompassed only samples giving very low signals in the assay, reflecting a distribution of results negative for parvovirus-specific IgG.

Fig. 1. Frequency distribution of parvovirus B19 serological data stratified by age group.
Of 2835 samples screened in this study, 808 (28·5%) gave a signal that lay beyond the measurement threshold of the limit of the detection system used (OD⩾3·0). The majority (53·8%) were found in samples from those aged 15–34 years. The quantitative data are therefore right-censored, and were accounted for by using an appropriate statistical model for subsequent quantitative analysis. This also denotes that the precise shape of the distribution of quantitative results arising from samples providing strong signals in the assay is uncertain, particularly its right tail. Thus, assuming that the component distribution with the lowest mean reflected samples with no evidence of parvovirus B19-specific antibody and the remaining two represented those with evidence of specific antibody, a fixed cut-off was selected that provided a PPV of 95%, assuming an overall prevalence of at least 50% in the sample population screened (Fig. 2). Figure 1 shows the position of both this fixed cut-off and that recommended by the assay manufacturer relative to the frequency distribution of quantitative results.

Fig. 2. Positive predictive values (PPVs) for different cut-offs (reactivity groups) for a variety of assumed overall parvovirus B19 prevalence estimates (dashed vertical line shows the lowest cut-off giving a PPV⩾95% with prevalence ⩾50%).
The multivariable analysis considering results (n=2835) categorized qualitatively as either positive or negative for parvovirus B19-specific IgG using this fixed cut-off showed no association of antibody prevalence with sex (P=0·2) or region (P=0·1). However, there was evidence of a strong association with age that increased with age in a nonlinear fashion (P<0·001) (Fig. 3).

Fig. 3. Age-specific parvovirus B19 antibody prevalence estimates (95% CI).
A total of 1596 (56·3%) sera were considered as having evidence of parvovirus B19-specific IgG using the fixed cut-off identified with the assistance of the mixture model. The censored normal regression model used to further investigate these data quantitatively showed a strong nonlinear association with age (P<0·001). Compared to those aged 1–4 years, antibody levels were highest in those aged 15–34 years, being 4·63 times higher (P<0·001, 95% CI 3·30–6·49). Antibody levels in those aged 5–14 years and ⩾35 years were similar, being 2·60 times higher (P<0·001, 95%CI 1·83–3·70) and 2·56 times higher (P<0·001, 95% CI 1·80–3·64) respectively in comparison to levels found in those aged 1–4 years. Quantitatively there was also an association between IgG levels and sex, with levels in females being 28·5% higher compared to those in males (P=0·004, 95% CI 8·2–52·6). Further investigation showed there to be weak evidence of an interaction between age and sex (P=0·0495), but no trend could be identified. There was no evidence that results varied by region when analysed quantitatively (P=0·247).
The model used to estimate the average annual force of infection in each of the age groups fitted the serological data well (deviance 12·9 on 5 d.f.). The best estimate for the average annual force of infection in those aged 1–4 years was 0·087 (95% CI 0·071–0·103), 0·011 (95% CI 0–0·049) in those aged 5–9 years, 0·109 (95% CI 0·072–0·136) in those aged 10–14 years, and 0 (95% CI 0–0·0068) in those aged 15–44 years.
Applying the upper 95% confidence interval for the force-of-infection estimate in those aged 15–44 years to population data and maternity estimates for England and Wales obtained in 1996 suggests an average of up to ∼26 500 parvovirus B19 infections may occur each year in females aged 15–44 years, of which up to 1257 could be in pregnant women (equating to one parvovirus B19 infection in every 512 pregnancies annually). Assuming that the risk of fetal death following a maternal infection during the first 20 weeks of pregnancy is 9%, and the risk of hydrops fetalis following maternal infection between 9 and 20 weeks of gestation is 2·9%, up to 59 fetal deaths and 11 cases of hydrops fetalis are estimated annually (see Table).
Table. Estimated average age-specific annual totals of all parvovirus B19 infections, infections during pregnancy, fetal deaths and cases of hydrops fetalis expected in females aged 15–44 years in England and Wales

The 95% confidence interval for the average annual force of infection was used (0–0·0068) and the risk of fetal death (weeks 1–20) and hydrops fetalis (weeks 9–20) taken as 9% and 2·9% respectively. Mid-1996 age-specific population and maternity estimates were provided by www.statistics.gov.uk
Figure 4(a, b) shows all available laboratory confirmation data for parvovirus B19 received for the years 1993–2005, stratified by year, sex and age. In total, 18363 laboratory confirmations were obtained during this period, with the majority (82·3%) received during the epidemic periods of 1993–1994, 1997–1998 and 2002–2003. In total 82·6% were from females, of which 80·9% were from those aged 15–44 years. Overall 89·3% of all laboratory-confirmed cases of parvovirus B19 were from persons aged ⩾15 years, with a total of 12 269 obtained in women aged 15–44 years, giving a mean of 944 laboratory-confirmed cases per year in females in this age group. A total of 8·6% of females of childbearing age with a laboratory-confirmed parvovirus B19 infection had information in a comments field that identified them as being pregnant.

Fig. 4. (a) Total available laboratory-confirmed cases of parvovirus B19 infection, stratified by year and sex (□, females; ■, males). (b) Laboratory-confirmed cases of parvovirus B19 infection in females only, stratified by year and age group (□, 0–14 years; ■, 15–44 years;![]() , ⩾45 years).
, ⩾45 years).
DISCUSSION
The age-stratified antibody prevalence estimates for parvovirus B19 obtained in this study reflect those of earlier studies [Reference Zaaijer, Koppelman and Farrington2, Reference Gay3, Reference Kelly5, Reference Cohen and Buckley6], suggesting the epidemiology of this infection has not changed with time. Prevalence increases nonlinearly with age, the majority of infection being acquired in those aged <15 years, reflecting that most parvovirus B19 infection occurs during childhood with very little occurring in the adult population. However, from a clinical and public health perspective it is infection in adults that is of concern, particularly amongst females of childbearing age where there is a risk of fetal mortality and hydrops fetalis if infection is acquired during pregnancy.
The force of infection will change during the course of an epidemic cycle, being highest during an epidemic period, which occurs every 3–5 years. Here the mean value is calculated over both epidemic and non-epidemic periods, although the majority of infection will be found during epidemics of parvovirus B19. Although the point estimate for the force of infection in those aged 15–44 years is zero this is unlikely to be correct since there are laboratory confirmations of parvovirus B19 infection in this age group. However, the upper 95% confidence interval provided by this study is consistent with estimates previously made for this age group [Reference Zaaijer, Koppelman and Farrington2, Reference Gay3], indicating that <1% of those susceptible in this age group become infected. Using this upper 95% confidence interval estimate for the force of infection, estimates of incidence of total parvovirus B19 infection and maternal infection in females of childbearing age are therefore comparable to those of previous studies. However, the incidence of hydrops fetalis has not been estimated before in England and Wales, and the previous estimate of the incidence of fetal deaths resulting from maternal parvovirus B19 infection applied a risk of 9% throughout the whole pregnancy [Reference Gay3, 10]. A subsequent larger study confined this risk of fetal death to maternal infection acquired between weeks 1–20 of gestation, and also estimated the risk of hydrops fetalis to be 2·9% as a result of maternal infection between weeks 9–20 of gestation [Reference Miller4]. Using these more recent data we therefore estimate an annual average of up to 59 fetal deaths and 11 cases of hydrops fetalis as a result of maternal parvovirus B19 infection in England and Wales when applying the upper 95% confidence limit for the estimate of force of infection obtained from the serology data used in this study. Whilst the estimate of fetal death as a result of maternal parvovirus B19 infection in pregnancy is somewhat lower than that previously made, these data still highlight that maternal parvovirus B19 infection is likely to remain a significant public health concern with up to 295 fetal deaths and 55 cases of hydrops fetal possibly occurring during an epidemic year.
Parvovirus B19 infection is not a notifiable disease in the United Kingdom and analysis of the laboratory confirmation data suggests they do not reflect the epidemiology of this disease in the general population as suggested by the antibody prevalence data. Most laboratory-confirmed cases come from women of childbearing age and caution should therefore be exercised when using laboratory confirmations to assist with investigating the epidemiology of this disease. Comparison with analysis of the antibody prevalence data also suggests laboratory confirmations reflect only a fraction of the upper estimate of 79 000–130 000 cases of parvovirus B19 infection in women of childbearing age suggested by the serology that could occur during an epidemic. Provision for collecting data on pregnancy status is made using a comments field, although the accuracy with which this information is recorded should also be questioned since only 8·6% of women of childbearing age could be identified as being pregnant. However, if it is assumed that the majority of cases confirmed in females aged 15–44 years are pregnant mothers then laboratory confirmations begin to approach the upper estimate of 3600–6000 maternal infections expected during an epidemic period. This suggests that parvovirus B19 infection in females of childbearing age may be under-diagnosed with a lack of information on pregnancy status. This highlights the need for enhanced surveillance of rash fever illness in this group, including collecting accurate and reliable information on pregnancy and outcome of the pregnancy, enabling the public health implications of this condition to be more accurately understood.
Consistent with previous antibody prevalence studies for viral infections in an unvaccinated population [Reference Gay3, Reference Gay9, Reference Vyse12], data obtained from this study distributes into discrete populations that may be considered to represent those susceptible and those with evidence of prior infection with parvovirus B19. This can be justified by considering the mean values of each of the three normal distributions used to model the data. One had a considerably lower mean than the other two, and encompassed only those samples giving very low signals in the assay used suggesting this population had no evidence of specific IgG. The remaining two distributions had comparatively much higher mean values and were therefore considered to represent samples containing specific IgG [Reference Vyse13].
These quantitative data contrast with the only previous large population study carried out in the United Kingdom. This used the MRL assay and the distributions were much more obscured making it difficult to distinguish between positive and negative specimens [Reference Gay3, Reference Gay9]. This suggests that the Mikrogen assay is more sensitive and can better differentiate between those with evidence of specific IgG from those without. However, as has been found in previous population prevalence studies, a consideration of the manufacturer's fixed cut-off relative to the frequency distribution of quantitative data shows it will lead to an underestimate of prevalence at the population level, as it is biased towards specificity at the expense of sensitivity for individual diagnostic use in a clinical setting [Reference Vyse12, Reference Vyse13]. This study therefore reinforces that categorizing results of population prevalence studies to accurately differentiate between those exposed to a particular infection from those unexposed is not always straightforward, and an approach that considers the distributions of quantitative data is likely to yield a more accurate interpretation at the population level [Reference Vyse12, Reference Vyse13].
A large number of samples used in this study provided a result that was right-censored, indicating the shape of the right tail of the frequency distribution considered to represent samples with high titres of specific IgG is not known. The mixture model used to model the frequency distribution of quantitative results was only therefore used to assist with setting a suitable fixed cut-off point for categorizing data, and further inferences from it were not made. That a large proportion of samples gave a signal beyond the measurement threshold of the detection system used suggests that the human immune system may make a particularly strong IgG response to parvovirus B19 infection, although it could also reflect a limitation of the instrument used.
Symptoms of parvovirus B19 infection, which include rash and arthralgia, are thought to be caused by the antigen antibody complex rather than the disease itself and are possibly related to the strength of antibody response [Reference Cohen and Mortimer1, Reference Gay3, Reference Woolf14]. Quantitatively, this study found antibody levels to be highest amongst those aged 15–34 years. This may reflect boosting through contact with infected children and/or younger siblings with the infection.
This study also found evidence that females make a stronger IgG response to parvovirus B19 infection compared to males. A previous study reported that acute arthropathy as a result of B19 infection is more frequent and persistent in adults, particularly amongst women [Reference Woolf14]. This complements the findings of this study that IgG levels are highest in young adults, and are also more likely to be higher in females than males. However, further studies are needed to ascertain the proportion of cases that are symptomatic amongst adults compared to those in children.
Gender differences in response to infectious disease have previously been reported for hepatitis B virus (HBV). Females have been found to be more likely to produce anti-HBs in response to infection compared to males [Reference Blumberg15] and it has been observed that female children respond to recombinant hepatitis B vaccine with a higher titre than males [Reference Fang16]. There is therefore some evidence that the female immunological response to infectious disease may differ from that of males, although reasons for this are unclear. It could be hypothesized that females have evolved over time to produce a stronger IgG response as during pregnancy this IgG is shared with the developing fetus. However, further studies are needed to investigate gender-specific immunological responses in more detail and should be extended to other commonly acquired infections.
ACKNOWLEDGEMENTS
We thank Professor Elizabeth Miller (Health Protection Agency, Centre for Infections) for valuable discussion and comments on the final draft of the manuscript. Funding for this study was provided by POLYMOD (EU FP6, Contract no. SP22-CT-2004-502084).
DECLARATION OF INTEREST
None.