INTRODUCTION
Carnivores have been shown to be reservoirs of a wide range of Bartonella spp. [Reference Chomel and Kasten1]. Worldwide, various wild canids have been identified as natural reservoirs of Bartonella spp. In North America, Bartonella spp. have been isolated from coyotes (Canis latrans), grey foxes (Urocyon cinereoargenteus), island foxes (Urocyon littoralis) and raccoons (Procyonis lotor) and serologically detected in American badgers (Taxidea taxus) [Reference Henn2–Reference Quinn4]. Bartonella have also been detected in river otters (Lontra canadensis) [Reference Chinnadurai5] and more recently in sea otters (Enhydra lutris) [Reference Carrasco6]. In Europe (France and the Basque Country, northern Spain) and Israel, B. rochalimae was isolated or detected from red foxes (Vulpes vulpes) and from a wolf (Canis lupus) [Reference Henn2, Reference Gerrikagoitia7]. A species close to B. clarridgeiae was detected in badgers (Meles meles) from the Basque Country, northern Spain [Reference Gerrikagoitia7]. In Great Britain, stoats (Mustela erminea) were reported to be commonly infected with Bartonella [Reference McDonald, Day and Birtles8]. In the Middle East, Candidatus B. merieuxii was detected in dogs and jackals from Iraq [Reference Chomel9]. In Asia, a new Bartonella sp. and one close to B. washoensis were isolated respectively from a Japanese badger (Meles anakuma) and a Japanese marten (Martes melampus) [Reference Sato10]. In Australia, B. clarridgeiae was detected in a red fox [Reference Kaewmongkol11]. Bartonella are usually vector-borne, with fleas and possibly ticks being the most likely vectors in canids [Reference Breitschwerdt12]. Wild carnivores, including wild canids, are hosts or potential hosts for numerous species of Bartonella, yet little research has been done to quantify their infection rates in South America. We sought to investigate Bartonella seroprevalence in captive wild canids from 17 zoos in São Paulo and Mato Grosso states, Brazil. Blood samples were collected from 97 wild canids belonging to four different native species and three European wolves [Reference André13].
MATERIAL AND METHODS
Animals
A total of 5 ml of blood was collected from each of 97 Brazilian wild canids belonging to four different species (Table 1), including 27 bush dogs (Speothos venaticus), 39 crab-eating foxes (Cerdocyon thous), 23 maned wolves (Chrysocyon brachyurus), eight hoary foxes (Lycalopex vetulus) and three European wolves (Canis lupus), maintained in captivity in 17 Brazilian zoos of São Paulo and Mato Grosso states [Reference André13]. All samples were collected under IBAMA license numbers S02027.002943/2005 and 15901–1. Animals were immobilized with a mixture of ketamine (Francotar®, Virbac, France) (10 mg/kg) and xylazine (Francotar) (1 mg/kg). Physical examination showed that sampled animals were apparently healthy. No ticks were found on any of the sampled animals.
Table 1. Number, species, and zoological park location of sampled wild canids, Brazil
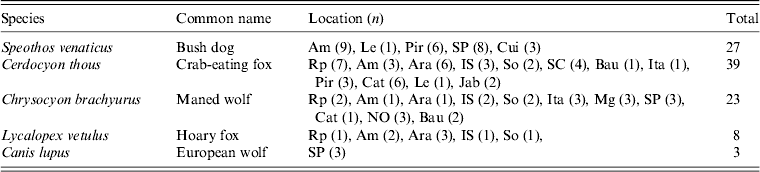
AMC, Association Mata Ciliar, Jundiaí, SP; Am, Americana Zoo, SP; Ara, Araçatuba Zoo, SP; Bau, Bauru Zoo, SP; Cat, Catanduva Zoo, SP; Cps, Campinas Zoo, SP; Cui, Cuiabá Zoo, Mato Grosso; IS, Ilha Solteira Zoo, SP; Ita, Itatiba Zoo, SP; Jab, Jaboticabal Zoo, SP; Le, Leme Zoo, SP; Mg, Mogi Mirim Zoo, SP; NO, Nova Odessa Zoo, So, Sorocaba; SP; Pir, Piracicaba Zoo, SP; Rp, Ribeirão Preto, SP; SC, São Carlos Zoo, SP; SP, São Paulo Zoo, SP.
Serology
Antibodies against B. vinsonii subsp. berkhoffii, B. clarridgeiae, B. henselae and B. rochalimae were detected using an indirect immunofluorescent antibody assay (IFA). These four antigens were selected, as B. henselae, B. vinsonii subsp. berkhoffii and B. rochalimae have been frequently detected in domestic dogs as well as B. clarridgeiae, which can also be a good substitute for detection of B. rochalimae [Reference Namekata14, Reference Schaefer15]. The IFA procedure was similar to a procedure described previously [Reference Henn16], with the following modifications. A 90% confluent tissue culture flask (containing MDCK cells) was inoculated with a 4-day-old culture of B. vinsonii subsp. berkhoffii (ATCC 51672) resuspended in 0·5 ml saline. Similarly, flasks containing Vero tissue cultures were inoculated with B. clarridgeiae (ATCC 51734), B. rochalimae (ATCC BAA-1498) or a mixture of B. henselae (ATCC 49882) and B. henselae U4 (University of California, Davis, strain). Drops (40 μl) of the infected tissue culture were spotted onto 12-well glass slides (Cel-Line®, Thermo Scientific, USA), the tissue culture allowed to adhere overnight, and the slides were then washed in PBS and fixed with acetone for 20 min. Serum samples added to the test wells were screened at 1:64 dilution in PBS with 5% milk. Slides were incubated at 37°C for 30 min, followed by three washes in PBS. Fluorescein-conjugated goat anti-dog immunoglobulin G (IgG; ICN Biomedicals Inc., USA) was diluted in PBS (1:1400 for B. vinsonii subsp. berkhoffii, 1:3600 for B. clarridgeiae and B. rochalimae and 1:2800 for B. henselae) with 5% milk containing 0·001% Evans Blue, and 20 μl of the dilution was applied to each well. The slides were incubated at 37°C for 30 min and again washed in PBS three times. The intensity of bacillus-specific fluorescence was scored subjectively from 1 to 4. Samples with a fluorescence score of ⩾2 at a dilution of 1:64 were reported as positive and final titration was performed (last dilution with a score ⩾2). The same two readers performed a double-blind reading of each slide. Negative and positive control samples were included on each slide.
RESULTS
Overall, Bartonella antibodies were detected in 11 (11%) of the canids, including five (12·8%) of the 39 crab-eating foxes, three (11·1%) of 27 bush dogs, two (8·7%) of 23 maned wolves and one (12·5%) of eight hoary foxes, with titres ranging from 1:64 to 1:512 (Table 2). Antibodies against B. clarridgeiae were most frequently detected (82%, 9/11), whereas only four animals were seropositive for B. henselae (36·4%), two animals for B. v. berkhoffii (18·2%) and one for B. rochalimae (9·1%).
Table 2. Bartonella antibody titres for seropositive captive wild canids, Brazil
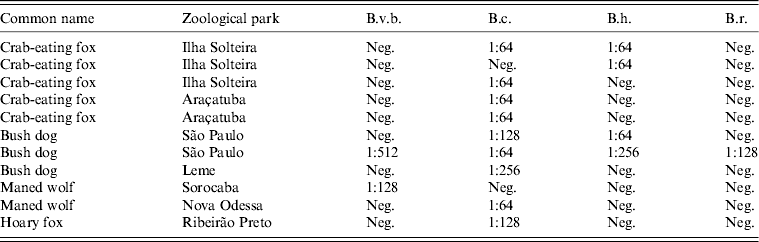
B.v.b., Bartonella vinsonii subsp. berkhoffii; B.c., Bartonella clarridgeiae; B.h., Bartonella henselae; B.r., B. rochalimae.
Two of the three seropositive bush dogs were from São Paulo Zoo (25%, 2/8) and the third one from Leme Zoo. The positive hoary fox was from Ribeirão Preto. The two seropositive maned wolves were from Nova Odessa and Sorocaba zoos, respectively, and three of the five crab-eating foxes were from Ilha Solteira (100%, 3/3); the two others being from Araçatuba (33%, 2/6). None of the three European wolves from São Paulo Zoo tested positive for Bartonella spp. One of the three seropositive bush dogs was seropositive for all four antigens, with the highest titre for B. v. berkhoffii. All three dogs were seropositive for B. clarridgeiae and only one of the three dogs was seropositive for B. clarridgeiae only (titre 1:256), which was also the case of the only seropositive hoary fox, one of the two seropositive maned wolves, but four of the five crab-eating foxes. The bush dog seropositive for two antigens was positive for B. clarridgeiae but also for B. henselae. The other maned wolf was only seropositive for B. v. berkhoffii. Two crab-eating foxes were seropositive for B. henselae, one of the two being also B. clarridgeiae positive.
DISCUSSION
We report the first evidence of presumptive Bartonella exposure in captive wild South American canids. Antibodies were detected in all four species, with an overall prevalence of 11% (range 8·7–12·8%, according to the canid species tested). Prevalence also varied depending of the location where these animals where in captivity from 0% to 100%. All animals tested were clinically healthy. Unfortunately, no specific information on age and sex of these animals was available regarding whether these animals were born in captivity or trapped in the wild. Bartonella are usually vector-borne, with fleas and possibly ticks being the most likely vectors in canids [Reference Breitschwerdt12]. It was recorded that none of the animals had ticks attached at time of examination, but no information on fleas were given. However, it is likely that if massive infestation had occurred, it would have been recorded.
In contrast to Northern America, limited information on infection of domestic dogs from South and Central America with various Bartonella spp. has been reported. The main data come from Brazil, Peru and Colombia, where dogs were found to be positive and/or seropositive by polymerase chain reaction (PCR) for B. v. berkhoffii, B. rochalimae and B. clarridgeiae. In Brazil, less than 3% of sick dogs from São Paulo region presenting at a veterinary teaching hospital were seropositive for B. v. berkhoffii or B. henselae and both species were detected by PCR in these dogs [Reference Diniz17, Reference Diniz18]. In stray dogs from São Paulo, seroprevalence was more than double (7·6%, 9/118) that reported in sick pet dogs presenting at a veterinary teaching hospital and 10·7% of 258 stray dogs from Bogota, Colombia [Reference Brenner19]. Both B. v. berkhoffii and B. rochalimae were detected by PCR in two of the seropositive dogs from Bogota. In Peru, a much higer seroprevalence was detected in free-roaming dogs from various Andean locations [Reference Diniz20], as seropositivity for B. rochalimae was detected in 67 dogs (62%), and for B. v. berkhoffii in 43 (40%) of the 108 dogs for which serum samples were available. Reciprocal titres ⩾1:256 for B. rochalimae were detected in 19% of dogs, and for B. v. berkhoffii in 6·5% of dogs. Bartonella DNA was detected in 21 (10%) of the 205 dogs for which DNA was extracted. Fifteen dogs were infected with B. rochalimae, while six dogs were infected with B. v. berkhoffii genotype III. Out of 95 free-roaming dogs from Isabella Island in the Galapagos archipelago, Ecuador, 13 (13·7%) dogs were PCR positive, including eight dogs PCR positive for B. clarridgeiae, four dogs positive for B. elizabethae and one dog positive for B. henselae [Reference Levy21]. In the Caribbean islands, six (8%) of 73 dogs brought to the veterinary teaching hospital in St George, Grenada were seropositive for B. v. berkhoffii and Bartonella DNA was detected by PCR from one (1·4%) of these 73 dogs [Reference Yabsley22].
Our data widen the spectrum of Bartonella infection to four species of wild canids native of South America, as none of the three imported European wolves were seropositive. On the contrary, B. rochalimae was detected by PCR from a free-ranging wolf from northern Spain [Reference Gerrikagoitia7]. The captive South American wild canids were mainly seropositive to Bartonella spp. also endemic in domestic dogs in South and Central America. It is particularly interesting to note that most of our seropositive captive wild canids were positive (positive only for that antigen or with the highest titre) to B. clarridgeiae, which was also the species most commonly detected by PCR in dogs from Isabella Island in the Galapagos and the antigen for which dogs from Colombia had the highest prevalence and highest titres [Reference Brenner19, Reference Levy21]. Cross-reactivity between antigens is still possible; however, it was observed for only 3/11 seropositive animals and as it was a cross-sectional study, it is also possible that a given animal had been exposed to different Bartonella spp. In experimental infection of specific pathogen-free (SPF) dogs, cross-reactivity was not observed [Reference Balakrishnan23, Reference Chomel24]. Therefore, B. clarridgeiae, either of feline origin or closely related strains specific to canids could be highly prevalent in domestic dogs and wild canids in South America. Further investigation is warranted in free-ranging wild canids from this part of the world in order to better determine the Bartonella spp. for which they are natural or accidental hosts. Furthermore, it will be important to determine if these Bartonella spp. can cause endocarditis in domestic dogs and captive wild canids, as shown for domestic dogs in North America for B. v. berkhoffii, B. clarridgeiae, B. rochalimae and B. henselae [Reference Chomel25–Reference Fenimore28].
ACKNOWLEDGEMENTS
We thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the scholarship (07/59889-6) and financial support (08/55570-8); Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) for the concession of the license (S02027.002943/2005 and 15901-1) for collecting and packaging blood samples from wild canids; and Bosque Municipal Fábio Barreto de Ribeirão Preto, Zoológico de Americana, Zoológico de Araçatuba, Zoológico de Sorocaba, Zoológico de Leme, Zoológico de São Carlos, Zoológico de Itatiba, Zoológico de Mogi Mirim, Zoológico de Piracicaba, Zoológico de Nova Odessa, Zoológico de Catanduva, Zoológico de Cuiabá and Fundação Parque Zoológico de São Paulo.
DECLARATION OF INTEREST
None.





