INTRODUCTION
Globally, cancer of the oesophagus is the eighth leading cause of cancer mortality [Reference Ferlay1]. The US government spends an estimated $1.1 billion [2] on treating over 30 000 [3] Americans suffering from the disease each year. Barrett's oesophagus, the premalignant metaplasia of oesophageal tissue related to gastro-oesophageal reflux disease, is a major risk factor for oesophageal adenocarcinoma [Reference Morgan4]; however, the causes of squamous cell carcinoma are less clear.
Oesophageal squamous cell carcinoma (OSCC) has traditionally been associated with risk factors such as tobacco and alcohol [Reference Holmes and Vaughan5], although given the vast geographical differences in incidence, the aetiology of the disease remains poorly understood. Countries such as the People's Republic of China (China), Singapore, Iran, Chile, Brazil, South Africa and France have all been identified as high-risk regions for the development of OSCC where incidence rates are markedly higher than in other areas [Reference Syrjänen6]. Given this variance, many possible risk factors have been studied in an attempt to determine the cause of OSCC – including nutritional deficiencies (e.g. vitamins A, B, C) [Reference Syrjänen6], physical factors (e.g. poor oral hygiene, hot drinks) [Reference Kamangar7], chemicals (e.g. nitrosamines, tobacco, alcohol, opium) [Reference Kamangar7], and viruses [e.g. Epstein–Barr virus, herpes simplex virus, human papillomavirus (HPV)] [Reference Syrjänen6].
HPV was first described in 1949 in skin warts; however, it was not until the mid-1970s that it was linked to cervical cancer [Reference zur Hausen8]. The link between HPV and cervical cancer has since been well established, given global detection of HPV DNA in over 90% of cervical cancers and the lack of geographical variation [Reference Smith9]. Meanwhile, research was also being conducted into the malignant conversion of other anogenital as well as extragenital papillomatous lesions to squamous cell carcinomas [Reference zur Hausen8]. In 1985, specific HPV types were first found in oropharyngeal carcinomas and it is now thought that over 25–30% of these cancers may be caused by high-risk HPV types [Reference zur Hausen8]. In 1982, Syrjänen et al. published the first study into the histological presence of known HPV patterns in 60 Finnish patients with established OSCC and found positive results in 40% [Reference Syrjänen10]. Since then, research has continued into the possible causative role of HPV infection in OSCC pathogenesis.
The aim of this meta-analysis is to combine, examine and quantify the results from studies addressing the association between HPV infection and the development of OSCC on a global scale.
METHODS
Study protocol
We followed the PRISMA guidelines where possible in performing our systematic review [Reference Moher11]. A systematic search was undertaken through Medline (from 1950), PubMed (from 1946), EMBASE (from 1949) and Current Contents Connect (from 1998) and Google Scholar to 3 December 2012, to identify relevant articles where possible. The search used the terms ‘oesophageal cancer’ OR ‘esophageal cancer’ AND ‘human papillomavirus’, which were searched as text words and as exploded medical subject headings. The reference lists of relevant articles were also searched for appropriate studies. No language restrictions were used in either the search or study selection. There were four studies included which were not published in English [Reference Emadian12–Reference Kim15]. A search for unpublished literature was not performed.
Study selection
We included cross-sectional, case-control and cohort studies that met the following inclusion criteria: (1) all cases and controls were human and adult; (2) the oesophageal cancer was squamous cell carcinoma; (3) all patients had no other health conditions. We excluded studies that did not meet the inclusion criteria.
Data extraction
The data extraction was performed via a standardized data extraction form, collecting information on publication years, study designs, numbers of cases, numbers of controls, total sample sizes, countries, continents, development statuses, control sources, mean ages, percentage male of total samples, the risk estimates or data used to calculate the risk estimates, confidence intervals (CIs) or data used to calculate CIs, the types of oesophageal carcinoma investigated, the methods of detection of HPV in the samples and the specific types of HPV detected. Where no data was available on the specific types of HPV detected, studies were included in the Non-specific analysis. Where no control group was used, studies were included in the Prevalence analysis. Quality of the studies was not assessed and authors were not contacted for missing data. Adjusted odds ratios (ORs) were extracted in preference to non-adjusted ORs; however, where ORs were not provided, unadjusted ORs and CIs were calculated. Where more than one adjusted OR was reported, we chose the OR with the highest number of adjusted variables. A sample which was found to be HPV-16 and HPV-18 positive was included in both subgroup analyses but in the overall analysis only once. However, a number of studies did not distinguish in their results between HPV-16 and HPV-18 positive samples and instead gave combined results as samples being positive for HPV-16/18. As such these studies were included in pooled HPV-16/18 analysis.
Four studies did not distinguish between histological types of oesophageal carcinoma and, in order to include them in the meta-analysis, they were assumed to be squamous cell carcinoma (Table 1). Where multiple control groups were studied, we selected the group of separate people over normal mucosal biopsies taken from areas adjacent to the tumour site.
Table 1. Summary of studies included in the meta-analysis
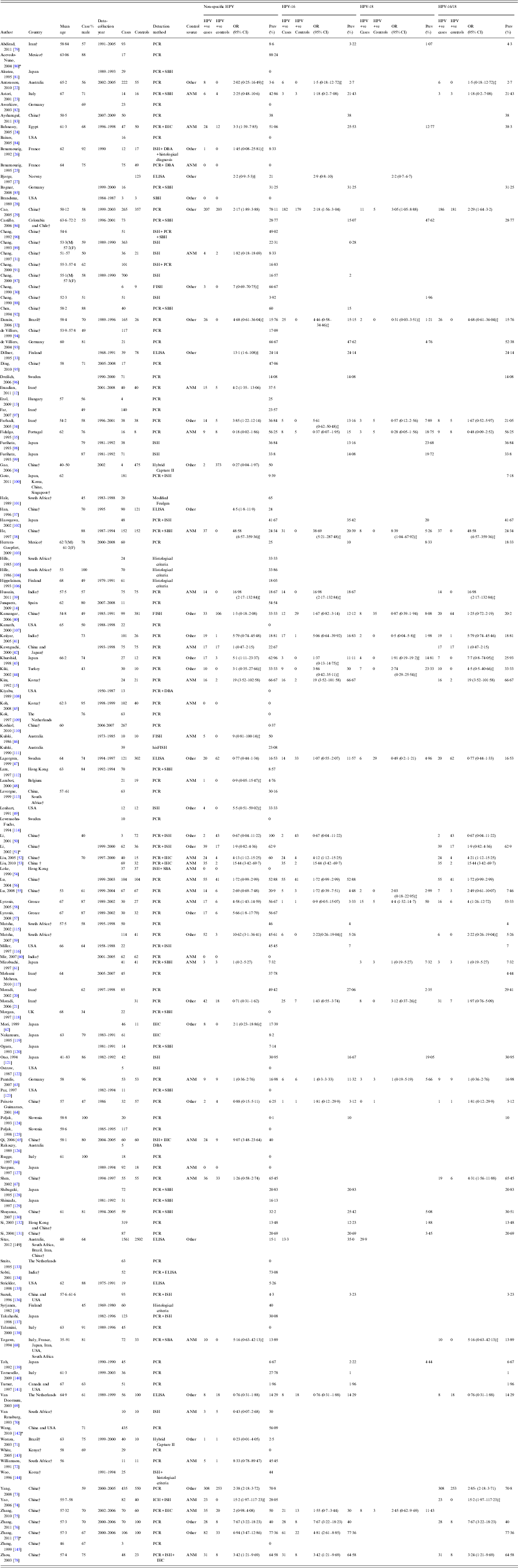
ANM, Adjacent normal mucosa; Other, other patients; PCR, polymerase chain reaction; SBH, Southern blot hybridization; ISH, in-situ hybridization; ELISA, enzyme-linked immunosorbent assay; FISH, fluorescence in-situ hybridization; ICH, immunohistochemistry; DBA, dot blot analysis; SBA, slot blot analysis; hisFISH, histopathological-fluorescence in-situ hybridization.
* All types of esophageal cancer.
† Classified as a developing country [Reference Government146].
‡ Insufficient data to calculate an OR as the number of HPV positive controls was 0 therefore in order to be able to calculate an OR, we added one HPV positive control sample.
§ Adjusted ORs – Bjorge et al. [Reference Bjorge27] adjusted for continine and Dillner et al. [Reference Dillner33] adjusted for smoking.
Statistical analysis
Pooled ORs and 95% CIs were calculated for the effect of HPV on the risk of developing OSCC using a random-effects model [Reference DerSimonian and Laird16]. We tested heterogeneity with Cochran's Q statistic, with P < 0·10 indicating heterogeneity, and quantified the degree of heterogeneity using the I 2 statistic, which represents the percentage of the total variability across studies which is due to heterogeneity. I 2 values of 25%, 50% and 75% corresponded to low, moderate and high degrees of heterogeneity, respectively [Reference Higgins17]. We quantified publication bias using Egger's regression model [Reference Egger18], with the effect of bias assessed using the fail-safe number method. The fail-safe number was the number of studies that we would need to have missed for our observed result to be nullified to statistical non-significance at the P < 0·05 level. Publication bias is generally regarded as a concern if the fail-safe number is less than 5n+10, with n being the number of studies included in the meta-analysis [Reference Orwin19]. We performed a sensitivity analysis based on sample size using different sample sizes (n < 10, n < 20, n < 50) and calculated the respective ORs. All analyses were performed with Comprehensive Meta-analysis version 2.0 (Biostat, USA).
Population attributable risk percentage (PAR%) was calculated using the formula:
The input data was taken from this meta-analysis.
RESULTS
From the literature search, we screened 1080 potentially relevant references, of which 444 abstracts were extracted for assessment (Fig. 1). Of these, 140 were selected for a detailed full-text review, and four studies were excluded – one included patients with tylosis, one studied HPV DNA presence in only p16-positive cases, one studied HPV DNA presence in only p16INK4-positive cases and one studied HPV DNA presence in OSCC with lymphoid stroma. There were five duplicate publications, we used the most recent in each case and the others were excluded. In total, 132 studies were included for analysis. There were two studies published by Moradi and colleagues [Reference Moradi20, Reference Moradi and Mokhtari-Azad21] that included the same case group; however, one study was a case-control study and was included in the analysis of risk while the other was a cross-sectional study and was used in the prevalence analysis. We excluded the case-control study [Reference Moradi and Mokhtari-Azad21] from the prevalence analysis due to repetition of data. In the event that the same authors published multiple studies within a similar time period, we used information from the Materials, Methods and Results sections to confirm that these were from different sample populations. Sixty-one studies had control groups comprising of normal tissue samples and were used in both the risk and prevalence analyses [Reference Emadian12, Reference Kim15, Reference Moradi and Mokhtari-Azad21–Reference Zhou78]. The 71 other studies contained either no control group or samples from controls with benign or structural oesophageal disease and were used only in the analysis of the prevalence of HPV DNA in OSCC [Reference Syrjänen10, Reference Erol13, Reference Junquera14, Reference Moradi20, Reference Abdirad79–Reference Zheng145]. In total, 13 795 oesophageal samples were included in the analysis, with 9291 as cases with established OSCC and 4504 as controls with normal oesophageal mucosa. The mean age of the patients was 59·36 (range of study means 45–67 years) and 70·1% were male (although data was only available in 82 studies) (Table 1).
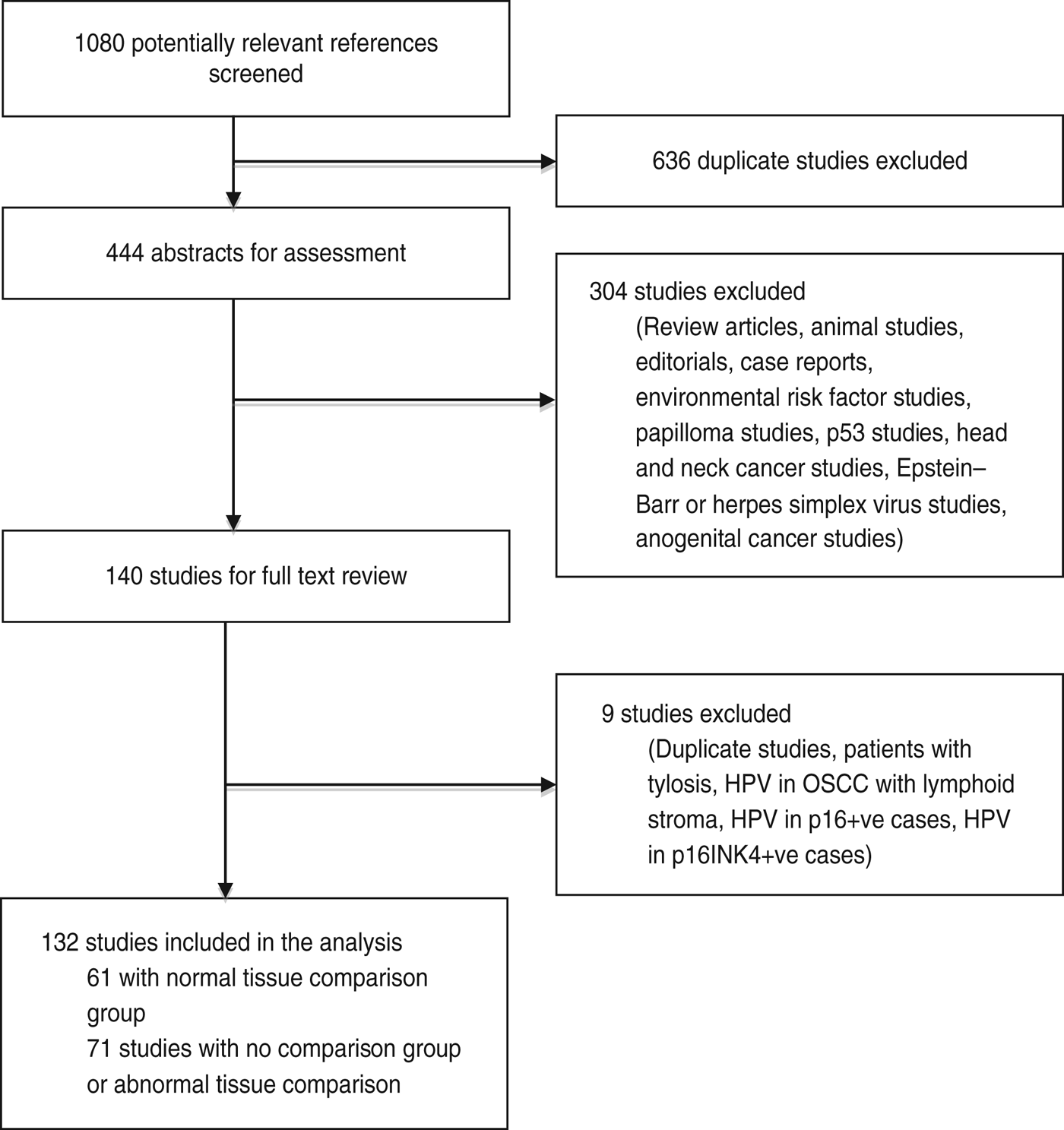
Fig. 1. Results of the literature search.
Prevalence
From the 138 studies that assessed prevalence (n = 12 037 cases), 27·4% (95% CI 21·2–28·8) of OSCC samples were infected with HPV (Table 2). These studies contained cases from 30 countries and six continents (Table 1), which during subgroup analyses, demonstrated geographical differences.
Table 2. Prevalence of human papillomavirus in oesophageal squamous cell carcinoma
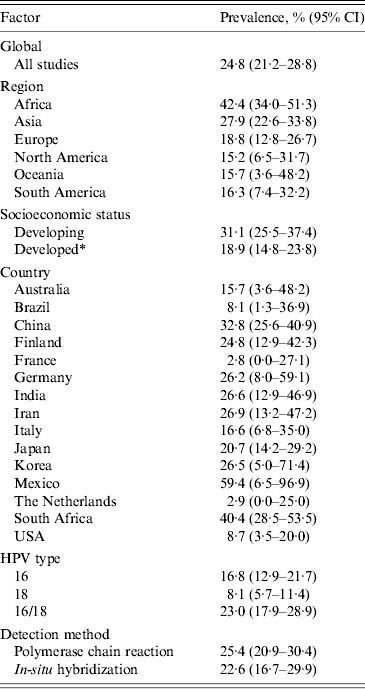
CI, Confidence interval.
* Countries classified as developing are identified in Table 1.
When comparing the prevalence according to economic development, developing countries had a 12·2% higher rate of HPV DNA in OSCC than developed countries [31·1 (95% CI 25·5–37·4) and 18·9 (95% CI 14·8–23·8), respectively]. We found that when analysing the prevalence by region, there was a 25·9% difference between the lowest, North America and the highest, Africa (Table 2). The largest population of people studied was Chinese (46 studies, n = 5859 cases), where the prevalence was found to be 32·8% (95% CI 25·6–40·9), ranking third highest globally behind only Mexico and South Africa (Table 2).
Of the 54 studies that reported data on the prevalence of HPV-16 (n = 4737 cases), 16·8% (95% CI 12·9–21·7) of cases contained HPV DNA, while of the 30 studies that reported data on HPV-18 (n = 2272 cases), only 8·1% (95% CI 5·7–11·4) of cases contained HPV DNA. However, 56 studies looked at high-risk HPV types, 16 and/or 18, (n = 4351 cases) and the combined prevalence was found to be 23·0% (95% CI 17·9–28·9).
There were 12 methods used to detect HPV in the samples (Table 1), the most commonly used being polymerase chain reaction (PCR) (94 studies) followed by in-situ hybridization (ISH) (24 studies), Southern blot hybridization (16 studies), immunohistochemistry (10 studies), enzyme-linked immunosorbent assay (8 studies), histological criteria (5 studies), dot blot analysis (4 studies), fluorescence ISH (3 studies), hybrid capture II (2 studies), slot blot analysis (2 studies), histopathological-fluorescence ISH (1 study), and the modified Feulgen technique (1 study). Thirty-seven studies used multiple detection methods, all of which included either PCR or ISH. In the 32 studies that included PCR, these were the results selected for analysis. In the remaining five studies, the ISH results were selected. The authors acknowledge that the use of histological criteria to diagnose HPV infection is a technique no longer employed. Two of the studies using histological criteria also utilized other detection methods in which cases the latter results were included; however, the remaining studies were included for a historical perspective.
Association between HPV and OSCC
Using the 61 studies (n = 3970 cases) that contained a normal tissue comparison group, we calculated a pooled OR of 2·69 (95% CI 2·05–3·54) (Fig. 2), demonstrating an increased risk of developing OSCC in the presence of HPV infection. There was a moderate level of heterogeneity (I 2 = 63·82%, P < 0·001), and with a fail-safe n of 1675 (P = 0·15) there was no evidence of publication bias (Fig. 3). We performed a number of subgroup analyses, looking at the risk of specific types of HPV, the effect of differing control sources, geographical variance, and the method of HPV detection (Table 3).

Fig. 2 [colour online]. Forest plot of association between the presence of human papillomavirus DNA and the development of oesophageal squamous cell carcinoma. There were six case-control studies that had no positive samples in either the case or control groups. Therefore these are not included in the forest plot.
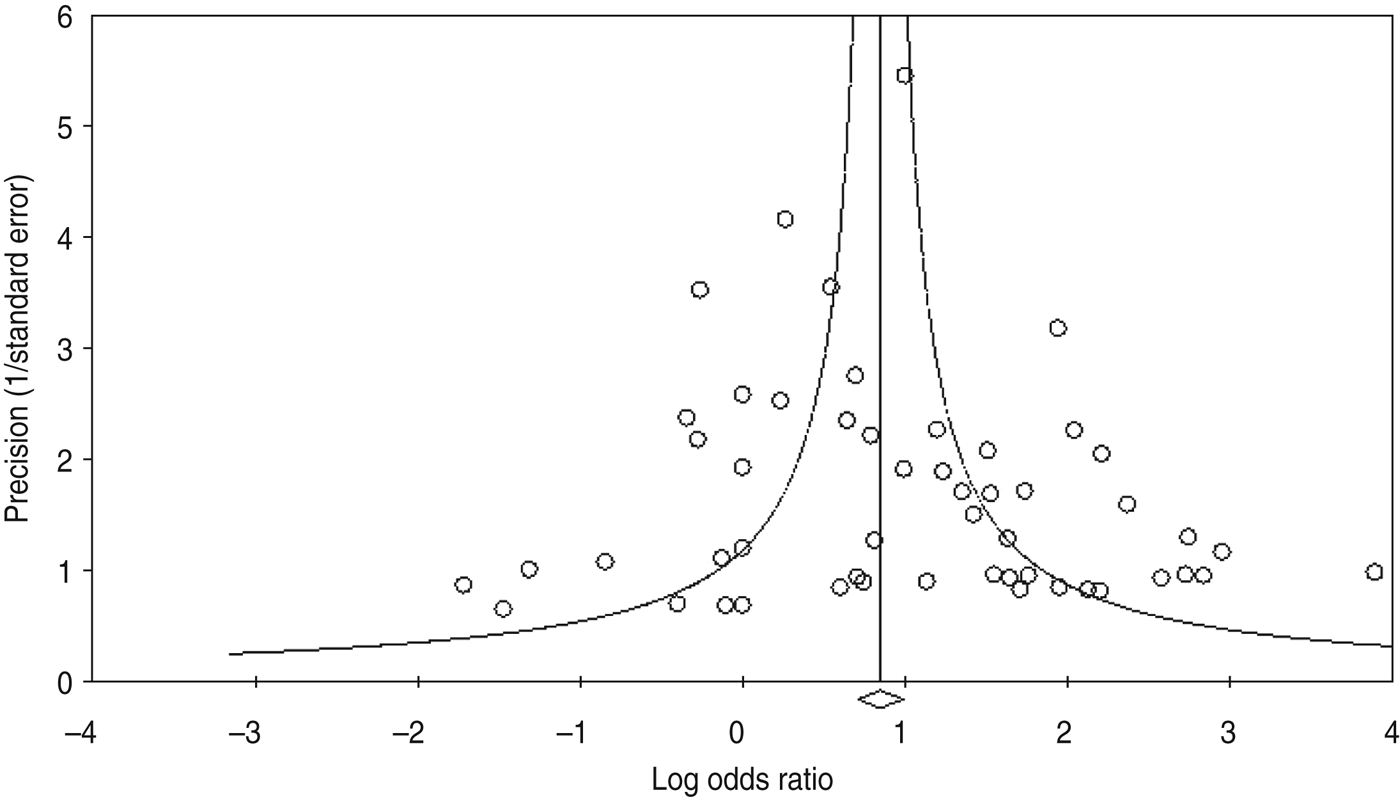
Fig. 3. Funnel plot of precision by log odds ratio.
Table 3. Subgroup analysis of case-control studies
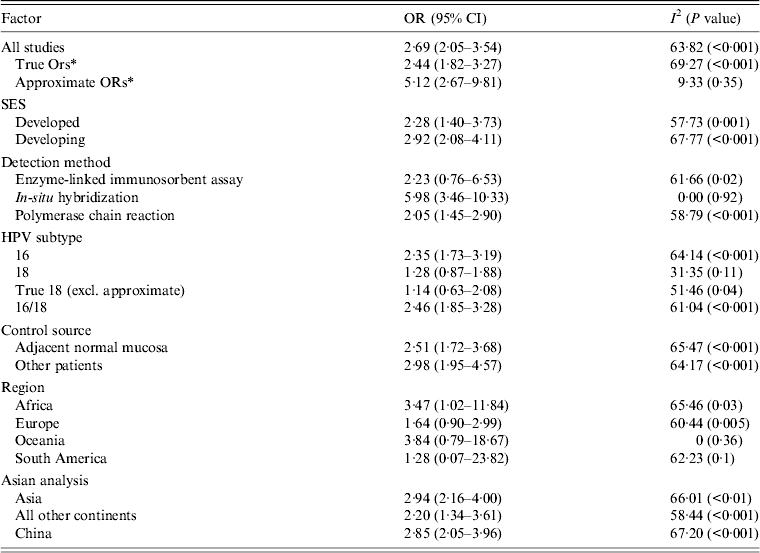
OR, Odds ratio; CI, confidence interval.
* Approximate ORs refers to case-control studies that had insufficient data to calculate an OR as the number of HPV-positive controls was 0 (Table 1). In order to be able to calculate an OR, we added one HPV-positive control sample. True ORs refers to case-control studies that had sufficient data to calculate an OR and therefore these are an absolute representation of the study.
There were 35 studies (n = 2942 cases) that provided information on specific types of HPV detected. We performed a subgroup analysis investigating the risk of HPV-16 and HPV-18, as well as combined HPV-16/18, in the development of OSCC. We found an increased risk of developing OSCC in patients with HPV-16 (OR 2·35, 95% CI 1·73–3·19) rather than HPV-18 (OR 1·28, 95% CI 0·87–1·88); however, a combined analysis of patients with HPV-16, HPV-18, or both resulted in a risk point estimate of 2·46 (95% CI 1·85–3·28). The heterogeneity was significantly high in all three analyses (Table 3).
Samples making up the control groups used in the studies were either biopsies of normal mucosa taken from an area of oesophgus adjacent to the tumour or from separate patients. We performed a subgroup analysis and found that studies that used separate patients as controls yielded a pooled OR of 2·98 (95% CI 1·95–4·57), which was higher than those studies that used adjacent samples (OR 2·51, 95% CI 1·72–3·68).
In concordance with the data assessing prevalence, there was a greater risk in developing countries of developing OSCC in the presence of HPV infection than in developed countries (Table 3).
Asia was the most predominantly studied region (36 studies, n = 2738 cases) and we calculated a risk point estimate of 2·94 (95% CI 2·16–4·0). There was a significant difference between this and the second most studied region, Europe (13 studies, n = 526), where the OR was found to be 1·64 (95% CI 0·90–2·99).
There were nine methods used in detecting HPV DNA in the samples (Table 1). PCR was the most commonly used method (41 studies), followed by ISH (10 studies), immunohistochemistry (8 studies), enzyme-linked immunosorbent assay (6 studies), Southern blot hybridization (4 studies), fluorescence ISH (2 studies), hybrid capture II (2 studies), dot blot analysis (2 studies) and slot blot analysis (2 studies). Sixteen studies used multiple detection methods, all of which included either PCR or ISH. In the 12 studies that included PCR, these were the results selected for analysis. In the remaining four studies, the ISH results were selected. We performed a subgroup analysis on the different detection methods, and studies using ISH resulted in the highest risk (OR 5·98, 95% CI 3·46–10·33), which was higher than the pooled risk of studies using PCR (OR 2·05, 95% CI 1·45–2·90).
PAR%
Using the formula discussed in the Methods section, the PAR% was calculated as a global figure as well as for differing regions. The results (Table 3) demonstrated that 15·7% of OSCC cases globally are attributable to being HPV positive. Regional analysis revealed a higher percentage in Asia (19·0%) than in Europe (6·3%). When analysed by socioeconomic status, developing countries were found to have a higher PAR than developed countries.
DISCUSSION
The results of our meta-analysis highlight an almost threefold increased risk of OSCC in the presence of HPV infection. The study population included cases from a range of geographical areas, high- and low-incidence areas for oesophageal cancer and high- and low-prevalence areas for HPV infection.
We found HPV-16 to be both the most prevalent type detected as well as the highest risk in terms of oncogenic potential. Of the studies with data on the various HPV types, we found HPV-16 to make up 65% of all HPV infections, and with an OR of 2·47 it was shown to be associated with the development of oesophageal cancer. HPV-16 infection was found to be twice as prevalent and almost double the risk for oesophageal cancer than HPV-18. Analysis of HPV-16 and/or HPV-18 showed that together these two types of HPV make up over 80% of HPV in infected samples, although the increase in risk is similar to that of HPV-16. These figures are in accordance with HPV infection in cervical squamous cell carcinoma, where HPV-16 is the most common type, followed by HPV-18 [Reference Smith9]. However, it has also been found that HPV-18 is the most predominant type found in cervical adenocarcinoma [Reference Government146], and this may also be the case for oesophageal adenocarcinoma although more research is needed to explore this potential link.
Control samples were either taken from normal oesophageal mucosa adjacent to the tumour site or completely different participants. A pooled subgroup analysis found studies using other participants to be have a higher risk than studies with adjacent normal mucosa as the normal control. One study included in our analysis, published by Benamouzig et al. in 1992 [Reference Benamouzig26], included two control groups, 17 separate patients with normal oesophageal musoca as well as 12 biopsies of normal mucosa taken from adjacent to the tumour site of the case patients. Out of the total 29 control biopsies, only one of the adjacent normal mucosa samples tested positive for HPV DNA by ISH. However, three such biopsies were found positive by histological diagnosis. This was hypothesized as possibly being due to paraneoplastic impairment of the local oesophageal mucosal immunity [Reference Benamouzig26]. As this effect can not be excluded, theoretically, separate patients as controls should yield a truer estimate of the risk. However, further research should be undertaken to evaluate this.
While Oceania and Africa were found to have the highest ORs, both groups had small numbers of studies (n = 2 and n = 4, respectively) and in terms of both the risk and prevalence analyses, the CIs overlapped considerably. This could be due to inter-region differences in baseline HPV prevalence or small sample sizes within the analysis. Therefore, these results are difficult to compare with regions such as Asia and Europe, which had much larger numbers of studies and while the CIs again overlapped, it was to a smaller degree. With this in mind, comparison of the results from Asia and Europe still demonstrated geographical variance. The risk for Asian populations was found to be higher than European populations. However, sample size may also play an important role again as it is important to note that the average sample size of cases in Asian studies is 76, compared to the average 40 of European studies. Interestingly, the geographical variance is in direct opposition to that of cervical cancer, which has a higher HPV prevalence in Europe, North America and Australia than Africa and Asia [Reference Smith9]. Given the questionable validity of these results without further research into general population prevalence of HPV as well as the inter-region differences from larger studies, it is difficult to interpret the significance of the geographical differences noted.
Previous research has shown that OSCC is the second most common cause of death in Chinese males [Reference He38]. Chinese studies made up a large proportion of our meta-analysis (35 studies, n = 3861 cases) and were found to have a pooled OR of 2·85. This put China at a greater risk than the global estimate of 2·69. The prevalence of HPV in OSCC in China was also found to be greater than the global estimate by 8%. A previous meta-analysis published in 2009 on the prevalence of HPV in OSCC in China found an average prevalence of 46·9% (95% CI 43·8–50·0) [Reference Li147], which is considerably higher than that of the present analysis, where the results showed a prevalence of 32·8%. However, the 2009 meta-analysis had a number of limitations – it included only 15 studies, compared to the 23 of this study, included studies only using PCR in the detection of HPV and excluded all studies that were not in Chinese.
This study attempted to analyse the general population prevalence utilizing the separate control group data; however, only 33 studies could be included (as opposed to normal mucosa adjacent to the tumour site) – less than a third of the included studies. On a global scale 22·8% of controls were HPV positive. Regional analysis demonstrated that the estimated general population prevalence in Asia was 35·2%, Europe 19·8%, Africa 7·7% and 0% in Oceania. Given the number of studies utilized to gain these results (Asia n = 19, Europe n = 3, Africa n = 2, Oceania n = 1), it is difficult to assess the reliability of these results. A wider study of the general population is required before an accurate prevalence of HPV can be determined.
Due to the multiple detection methods used, a subgroup analysis was also performed to evaluate for any differences. Previous research has shown that in terms of sensitivity of HPV detection, ISH is almost equivalent to PCR, and in terms of reproducibility it is the superior detection method [Reference Wiedorn148]. Therefore it is interesting to note that subgroup analysis showed that studies using ISH resulted in a pooled OR of 5·98 (95% CI 3·46–10·33), with minimal heterogeneity (I 2 = 0·00, P = 0·92); however, PCR studies resulted in a much lower risk (OR 2·05, 95% CI 1·45–2·90) with a much higher heterogeneity (I 2 = 58·79, p < 0·001). A possible explanation for this is the individual results in the ISH analysis as there were only seven studies included in the group, compared to the 41 in the PCR group.
The PAR% was also calculated based on data from this meta-analysis. The results showed that globally 15·7% of OSCC cases can be attributed to HPV. A limitation of this analysis is the use of the data from studies in this meta-analysis and a truer PAR% would be gained from using continent-wide statistics. This would require data on the incidence of OSCC throughout each region as well as oesophageal HPV positivity, which is not available at the current time. However, as a preliminary measure, our results demonstrate that 19·0% of OSCC cases in Asia can be attributed to HPV compared to 6·3% in Europe. Furthermore, Africa was found to have the highest PAR% at 28·7%; however, given the small number of studies from this region it is difficult to determine the significance – similarly for Oceania.
This meta-analysis has several strengths. A broad literature search was conducted with no language restrictions, a point which has been a limitation of previous meta-analyses on this topic. The search was also geographically unrestricted, which allowed for a broader insight into the global prevalence and risk associated with HPV and OSCC, as well as enabling the analysis of geographical variances.
This study also had several limitations. First, many of the studies included had small sample sizes – only 22 out of the total 131 studies had sample sizes over 100 cases. However, the risk ratios for different sample sizes (n < 10, n < 20, n < 50) were calculated and there was not statistically significant difference between them, suggesting that this had little impact on the combined estimates. Some analyses showed heterogeneity, which may have been due to small sample size. The use of adjacent normal mucosa as a control group also limited our ability to compare the results throughout the analysis. Only two of the studies provided ORs that had been adjusted for known risk factors for oesophageal cancer (i.e. alcohol and smoking), while the others provided only the raw data and unadjusted ORs were calculated. Therefore while this study was able to demonstrate an increased risk of OSCC in the presence of HPV infection, its independence as a risk factor is yet to be determined.
These results have demonstrated a considerable global association between the presence of HPV infection and the development of OSCC. They have implications for future research into the transmission of the virus to oesophageal mucosa as well as possible future treatment regimens for oesophageal cancer. On a regional scale, in areas such as Asia, where the incidence of OSCC is high, these results may have implications for immunization schemes and programmes advocating for preventative measures to minimize the transmission of the virus.
In conclusion, this study has confirmed a relationship between OSCC and HPV infection. In addition, it has demonstrated an increased risk in developing countries, particularly China, as well as with HPV-16 infection compared to HPV-18. The prevalence of HPV DNA in OSCC was found to be higher in similar areas, and HPV-16 found to be the most prevalent globally, with the highest risk in comparison to other HPV types.
DECLARATION OF INTEREST
None.








