Introduction
Shiga toxin-producing Escherichia coli (STEC) infections are a significant public health issue worldwide [Reference Majowicz1]. Circa 2010, STEC infections transmitted via food caused more than 1 million illnesses, 128 deaths and nearly 13 000 Disability Adjusted Life Years [Reference Kirk2]. Cost effective intervention to prevent such infections requires identifying the foods that are the most important vehicles of exposure.
To determine the specific food types associated with foodborne illnesses, different methods are used to investigate outbreaks vs. sporadic illnesses. In outbreak investigations, the goal is to identify the specific food exposure common across the cases and both retrospective cohort and case-control studies are used to meet this objective. For sporadic cases of illness, risk factors, including food types, are most commonly identified via case-control studies. In case-control studies, the association of cases with various food exposures can be quantified, typically through odds ratios (ORs) and meta-analyses of these studies may yield summary estimates for food exposures of interest [Reference Pires3]. Therefore, to inform future preventative action, the aim of this study was to determine the food types with the greatest association with sporadic STEC illness.
Methods
Review question/scope
The specific question addressed by this systematic review and meta-analysis was: what is the relative association of different foods with sporadic STEC illness? A search of the PROSPERO Registry, the Cochrane Library and PubMed revealed one potentially relevant systematic review and meta-analysis that examined the relative contribution of routes of exposure to STEC infection [Reference Kintz4]. Because this review: assessed broader routes of transmission (e.g. food, person to person); did not assess specific foods (other than raw/under-cooked meat); and only included larger (n ⩾ 20) studies of multiple designs (including but not limited to case-control studies), we determined that a new systematic review with a more in-depth analysis of different food categories was needed. The protocol for this review is registered in PROSPERO (# CRD42017074239; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=74239) and PRISMA guidelines were followed (except where formatting conflicted with journal requirements).
Eligibility criteria
The PECOS framework, as defined by Sargeant and O'Connor [Reference Sargeant and O'Connor5], was used to define the review eligibility criteria, as follows. The population was all human populations, with no limitations by age or other participant characteristics, location, or context/settings. The exposures were all foods (e.g. hamburger, leafy greens); we neither considered drinking water (tap, bottled, or other), breastfeeding, nor nasogastric feeding as foods, nor did we include studies that assessed general nutrition (including malnutrition) as a risk factor for STEC infection. The comparator group was individuals who are not ill with STEC infection (i.e. controls; determined either via laboratory testing or by the absence of symptoms) and the outcome was sporadic illness caused by laboratory-confirmed STEC infection. The study design was case-control studies and thus the effect measure of interest was the OR.
Search strategy
The search strategy was developed in consultation with a medical librarian and was reviewed by an expert in systematic reviews of foodborne disease, who was not involved in the original strategy development (IY). The search terms were developed through Medline Ovid and then adjusted as needed for each individual database searched. Details about search term development are given in the Supplementary Materials (Section A). A list of the final search terms is available via PROSPERO. The search was not limited by language, location, study period, or any other characteristics.
Searches were conducted from 01 August to 30 September 2017, in the following bibliographic databases: Medline (OVID), EMBASE, Scopus, CAB Direct, African Journals Online, Asia Journals Online and Latin America Journals Online. We also searched: the European Food Safety Authority (EFSA) journal; five databases of grey literature sources (ProQuest Dissertations and Theses, E-Theses Online Services (ETHOS), OpenGrey, Agricultural Research Service and Current Research Information System); the main World Health Organization (WHO) website, the six WHO regional websites, the Food and Agriculture Organization of the United Nations (FAO) website and the Africa Centers for Disease Control and Prevention website. To identify any unpublished or pre-publication studies, we consulted with: authors from Hooman et al. [Reference Hooman6] and Paudyal et al. [Reference Paudyal7]; WHO advisors from STEC-related reports identified on WHO websites; WHO regional public health contacts and members of the Joint FAO/WHO Core Expert Group on STEC/VTEC. Finally, we searched citation lists of all types of review articles on STEC identified during the search and the citation lists of our final set of references.
Citation collection, deduplication and screening
Citations were collected, managed, de-duplicated and screened in RefWorks (ProQuest LLC, 2017). Attempts were made to obtain English translations of articles in other languages; if suitable translation could not be obtained, the title and abstract were put through Google Translate for relevance screening and, if relevant, the entire article was reviewed and extracted by a native speaker of the article's original language with expertise in epidemiology and a familiarity with foodborne disease.
For initial relevance screening, titles and abstracts were screened by two independent reviewers per reference with a third reviewer to resolve conflicts. Inclusion criteria for relevance screening were the study is about STEC; a case-control study; done in humans; not an outbreak investigation. Citations fulfilling these criteria, or with insufficient information, were advanced to full-text screening, which was also completed by two independent reviewers per reference (with a third to resolve conflicts), using standardised instructions. Inclusion criteria for full-text screening, in addition to those above, were: the study investigates the exposures (or risk factors) experienced by a series of cases, compared to the exposures (or risk factors) experienced by a series of controls; the controls are not cases of some other disease; cases are individuals with illness caused by STEC; and, the study assessed food exposures. Studies that passed full-text screening advanced to data extraction. Further details about screening processes are given in the Supplementary Materials (Section A).
Data extraction and risk of bias assessment
Data were extracted by two reviewers, with a third reviewer to resolve conflicts, using standardised forms and instructions. A full list of the extracted variables, including how we assessed the appropriateness of the laboratory methods used, is given in the Supplementary Materials (Section A). Risk of bias was assessed using the Newcastle-Ottawa Quality Assessment Scale (http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf), modified to address critical items for case-control studies using relevant questions from the RTI International – University of North Carolina at Chapel Hill Evidence-based Practice Center Item Bank and its modifications [Reference Viswanathan and Berkman8,Reference Viswanathan9]. Overall study quality was captured using the RTI Overall Assessment question (‘are the results of the study believable taking study limitations into consideration’?), as well as a modified version of ROBINS-I, relevant to case-control studies [Reference Sterne10]. Finally, we assumed that age was the most important confounder given the established relationship between age and the risk of STEC infection [Reference Werber11e.g. ]. Thus, we assessed whether age was adequately controlled as part of our quality assessment by assessing the combined impact of the study's design and analyses.
Analysis
Data were stratified into different categories for analysis. Study countries were classified into WHO subregions [Reference Kirk2]. Food items were categorised using the United States' Interagency Food Safety Analytics Collaboration's hierarchical categorisation scheme of mutually exclusive food categories [Reference Richardson12]. In our registered protocol, we stated that raw/undercooked foods would be treated as separate items than cooked foods. However, given that many of the food items were reported with unknown raw/cooked status, we chose instead to group raw and cooked food items (e.g. categorize raw beef, cooked beef and beef of unknown status all as ‘beef’) and explore the impact of raw/cooked/unknown status in the meta-regression.
Descriptive analyses were conducted to summarise study characteristics. To calculate the individual, study-specific ORs for each food category for which results were reported, the following process was used. For all instances where the number of cases and controls who were either exposed or unexposed to a given food were reported in the paper directly, we used these exact values to calculate the OR and standard error (s.e.) post hoc. For the remaining instances where ORs were reported, we used the reported univariate OR and 95% CI to back-calculate the OR and s.e. To do this, we designed an optimisation process in which we fitted the log-transformed OR to a normal distribution and minimised the sum of squared differences between the observed and the fitted 95% CI. For comparison purposes, an alternate analytic approach was also applied, in which the reported univariate OR was used for all instances where such values were reported and the reported number of cases and controls who were either exposed or unexposed to a given food category was then used when ORs were not given. We used crude ORs and data, unadjusted for confounders because not all studies included adjusted ORs. All studies, regardless of characteristic, were included in the subsequent analyses.
Summary univariate ORs and their corresponding 95% CIs were calculated for each food category, both overall and by WHO subregion, using a random effects meta-analysis model, with restricted maximum likelihood used to weight studies. Heterogeneity was assessed using the I 2 statistic. Publication bias was assessed using the following: Begg and Mazumdar's rank correlation test [Reference Begg and Mazumdar13] and Egger's regression test [Reference Egger14]. When significant publication bias was present, we used Duval and Tweedie's trim-and-fill method [Reference Duval and Tweedie15] to explore the impact on model estimates. For the food categories with significant overall associations, meta-regressions were conducted to explore heterogeneity by examining the relationship between single study characteristics (i.e. WHO subregion, publication year, study population age and raw/cooked status) and the ORs for food exposures. For both the summary ORs and the meta-regressions, the effect of clustering by study was explored in a sensitivity analysis to ensure that a study with several food exposures in the same food category did not have inflated influence on the estimates [Reference Domingues16,Reference Domingues17]. To this end, we fitted multilevel meta-analysis models using the study as a random effect. All analyses were carried out in R using the ‘metafor’ package [Reference Viechtbauer18].
Results
Numbers of citations identified
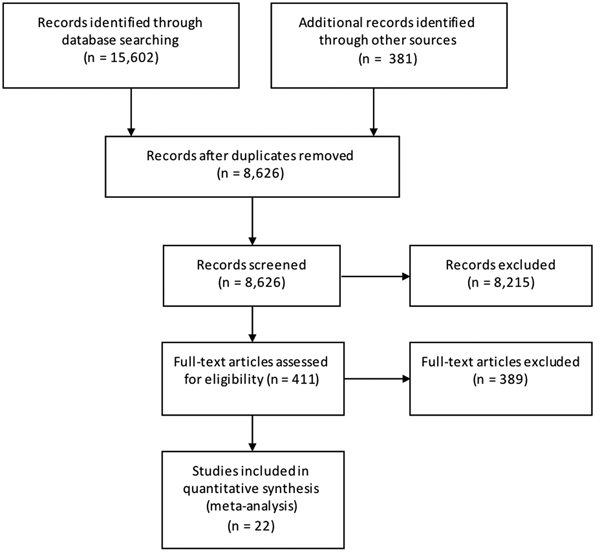
Results from the search, including the number of citations identified, are shown in Figure 1. The majority of the 411 full-text articles screened were in English, but 30 were in 13 other languages (Japanese, n = 9; Spanish, n = 7; Portugese, n = 3; French, n = 2; Czech, Chinese, Dutch, German, Hungarian, Italian, Romanian, Slovenian and Thai all n = 1). From these 411 articles, we identified 22 case-control studies of sporadic STEC infection in humans, from 10 countries within four WHO subregions (Region of the Americas A (AMR A) and B (AMR B), European Region A (EUR A) and the Western Pacific Region A (WPR A)), conducted from 1985 to 2012 (Table 1); study locations and timeframes are also shown in Figure 2. All 22 studies were published in English and were from the peer-reviewed, indexed literature [Reference Werber11,Reference MacDonald19,Reference Jaros39–].
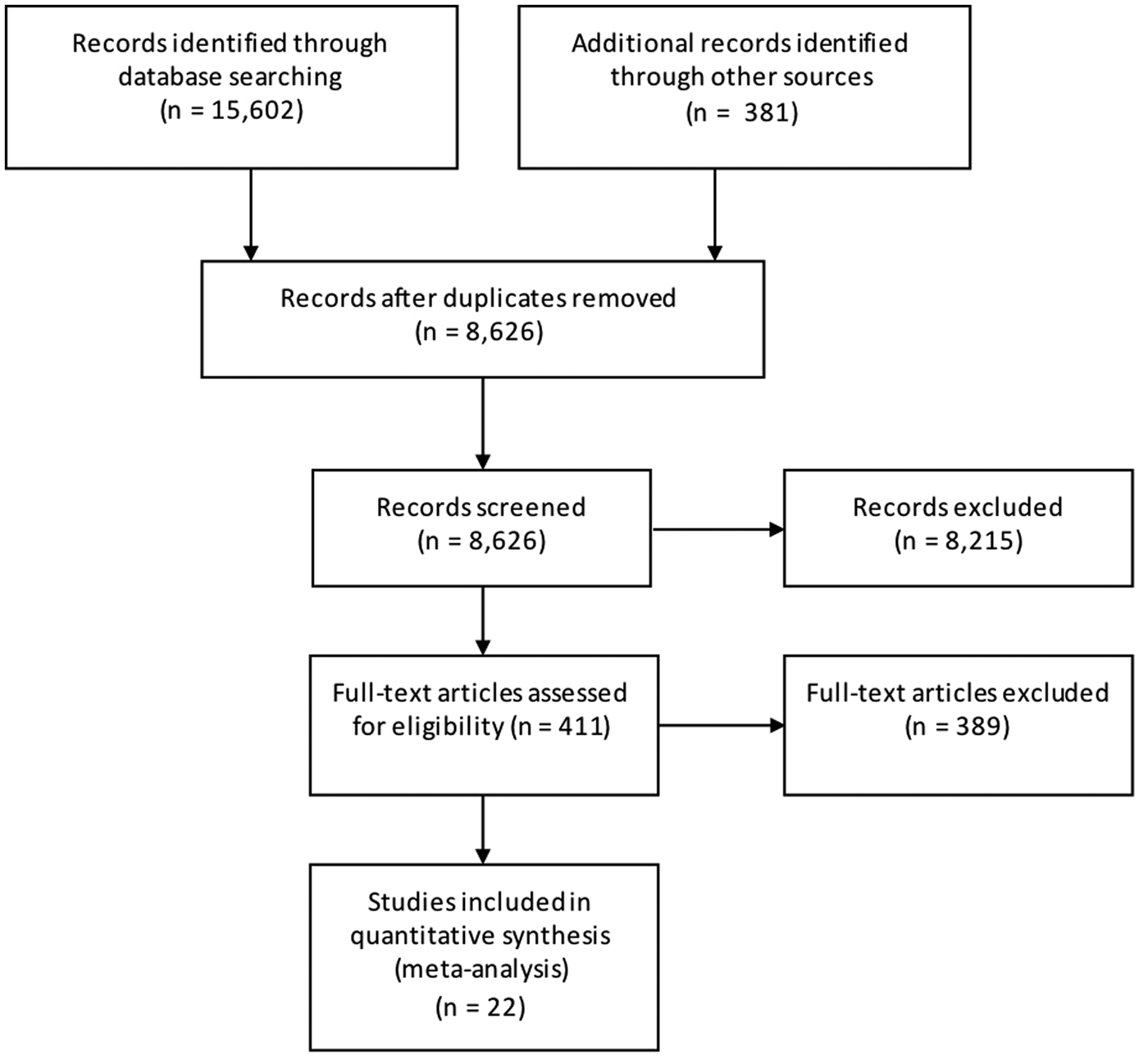
Fig. 1. PRISMA diagram showing the results of the search for case-control studies of sporadic STEC infections in humans (all dates and locations).
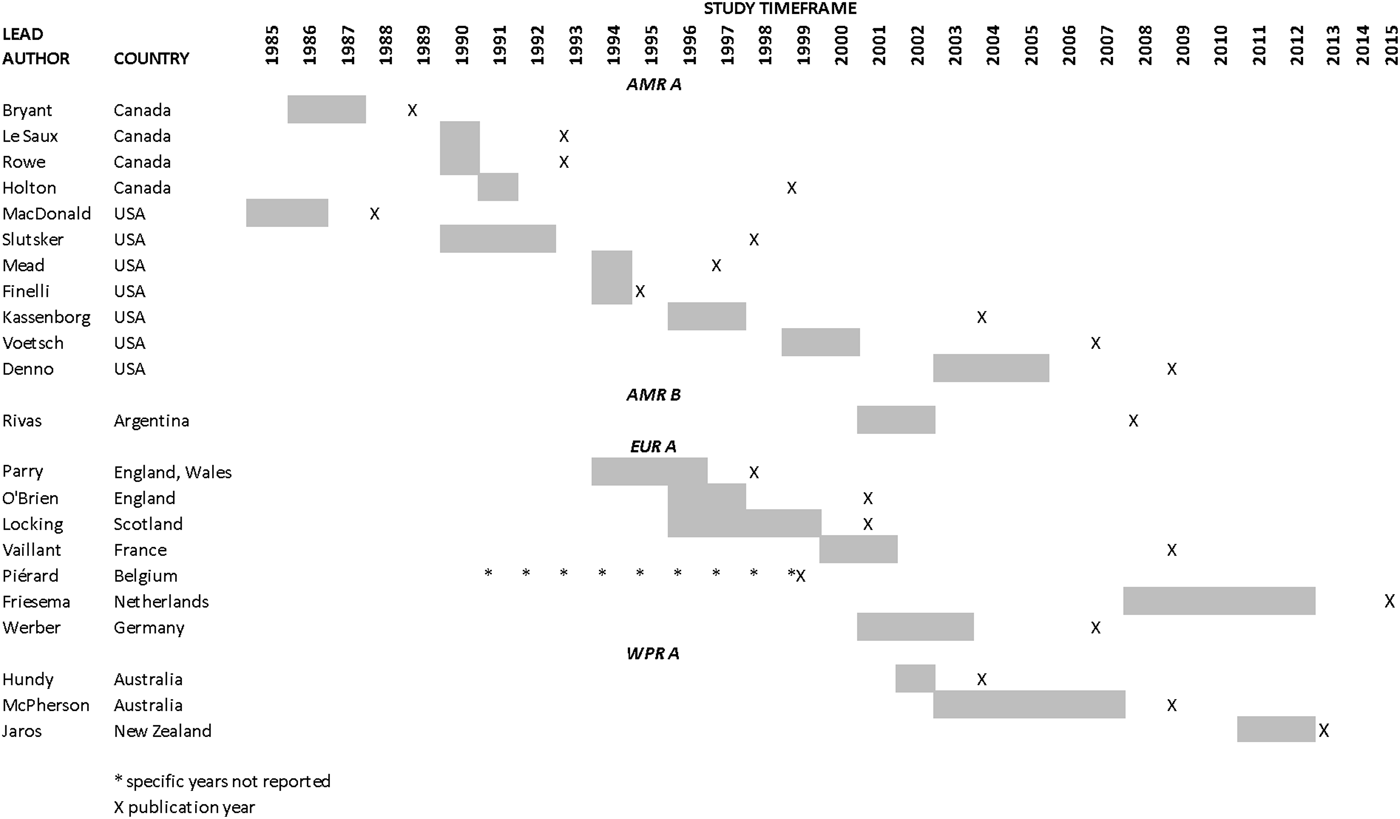
Fig. 2. Study locations and timeframes for the 22 identified case-control studies of sporadic STEC infections in humans.
Table 1. Characteristics of the 22 case-control studies of non-outbreak (i.e. sporadic) STEC infection in humans, ordered by study timeframe (oldest to newest)
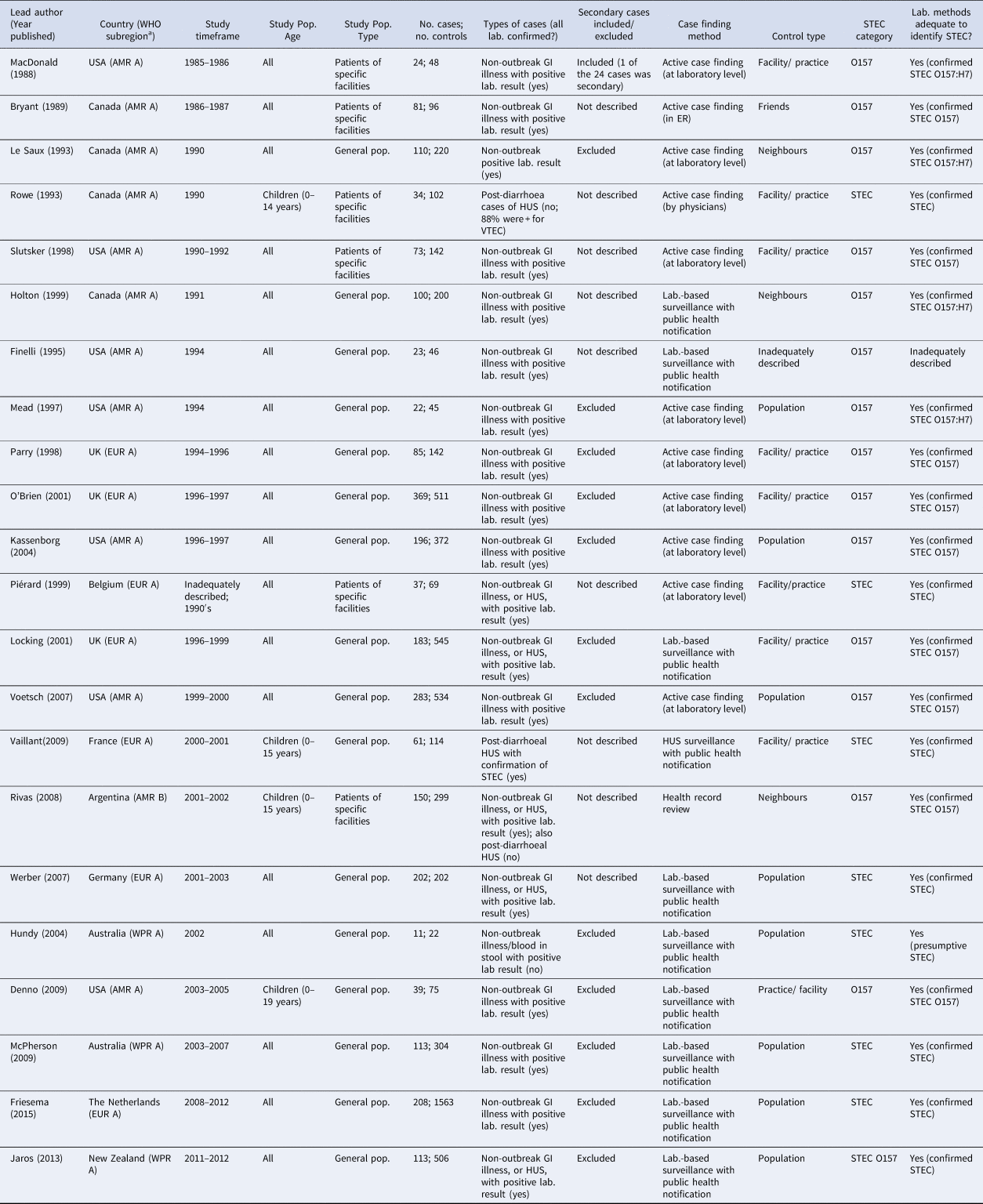
a WHO Subregions comprise the following countries: Region of the Americas A (AMR A): Canada, Cuba, USA.
Region of the Americas B (AMR B): Antigua and Barbuda, Argentina, Bahamas, Barbados, Belize, Brazil, Chile, Colombia, Costa Rica, Dominica, Dominican Republic, El Salvador, Grenada, Guyana, Honduras, Jamaica, Mexico, Panama, Paraguay, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago, Uruguay, Venezuela (Bolivarian Republic of).
European Region A (EUR A): Andorra, Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Monaco, Netherlands, Norway, Portugal, San Marino, Slovenia, Spain, Sweden, Switzerland, United Kingdom of Great Britain and Northern Ireland.
Western Pacific Region A (WPR A): Australia, Brunei Darussalam, Japan, New Zealand, Singapore.
Three potentially relevant studies were identified beyond the peer-reviewed literature databases. Two potentially relevant doctoral theses [Reference Kemp40,Reference Espie41] were identified in the grey literature search, but full-text documents were unavailable. Expert consultation identified a case-control study of sporadic human non-O157 STEC infections in the USA (Dr Patricia Griffin, US Centers for Disease Control and Prevention, Atlanta, GA; personal communication), but the results were unavailable at the time of our analysis.
Description of the identified studies
In terms of risk of bias, all studies used an adequate case definition, but for three of the 22 studies (14%) [Reference Rowe22,Reference Piérard30,Reference Rivas34], it was difficult to determine whether the cases were representative based on the information provided. Of the 22 studies, 12 (55%) excluded secondary cases of STEC, in one they were included and for nine studies, this information was not provided (Table 1). Of the 22 studies, 20 (91%) contained enough detail to demonstrate that the laboratory methods were adequate to identify STEC, one described the identification of presumptive STEC [Reference Hundy and Cameron35] and one did not provide adequate information to assess the laboratory methods used [Reference Finelli25] (Table 1).
Only one study [Reference Finelli25], published as a short report, did not provide an adequate description of control selection nor definition. All other studies used controls that were without symptoms of current gastrointestinal infection, rather than those with negative laboratory tests for STEC. Thirteen of the studies (59%) used different methods to identify cases vs. controls (Table 1); in all these studies, cases were identified via existing health system mechanisms, including laboratory-based surveillance, whereas controls were predominantly identified via random or semi-random sampling from the population. Considering feasibility, validity, ethical and other issues, control selection was considered appropriate in 21 of the 22 studies (96%; i.e. controls represent the population from which the cases arose and, if the controls had acquired STEC infection, they would have been included as cases in the study), with the exception of one study [Reference Finelli25].
Assessing how studies controlled for age in the study design, analysis, or both, two of the 22 studies (9%) [Reference MacDonald19,Reference Rowe22] did not appear to adequately control for age. Both these studies matched on age during control selection, but they did not account for this in their analysis. Of the 20 studies that adequately controlled for age, two (10%) did not match on age during control selection, but adequately adjusted for age by including it in their regression models [Reference Friesema38,Reference Jaros39] and the remaining 18 (90%) matched on age during control selection, as well as conducted analysis that accounted for matching on age.
In all studies, exposures were ascertained via interview using comparable questions for cases vs. controls. All studies assessed case exposures during the incubation period prior to illness, whereas for controls, half assessed control exposure during the window period prior to the control interview, while the other half assessed control exposure during the same calendar period as the cases (Table 2). Of the 10 studies that assessed control exposure during the same calendar period as the cases, only the three most recently published ones [Reference Voetsch32,Reference Rivas34–] reported the time elapsed between the exposure window and the case/control interview dates. In two of the three studies [Reference Voetsch32,Reference Rivas34] the time elapsed for cases and controls was comparable (⩽4 days difference), whereas in one study [Reference Vaillant33], controls were interviewed a median of 3 weeks later than cases were, suggesting the potential for differential recall bias. Non-response rates for cases and controls and descriptions of non-respondents were not given in most studies (17/22, 77%; Table 2). In 20 of the 22 studies (91%), the statistical methods applied were considered adequate to determine ORs for food exposures; in one study, there was insufficient information to make this assessment [Reference Finelli25] and in one study, the statistical methods were considered inadequate [Reference Rowe22].
Table 2. Selected risk of bias assessment indicators for the 22 case-control studies of non-outbreak (i.e. sporadic) STEC infection in humans, ordered by study timeframe (oldest to newest)
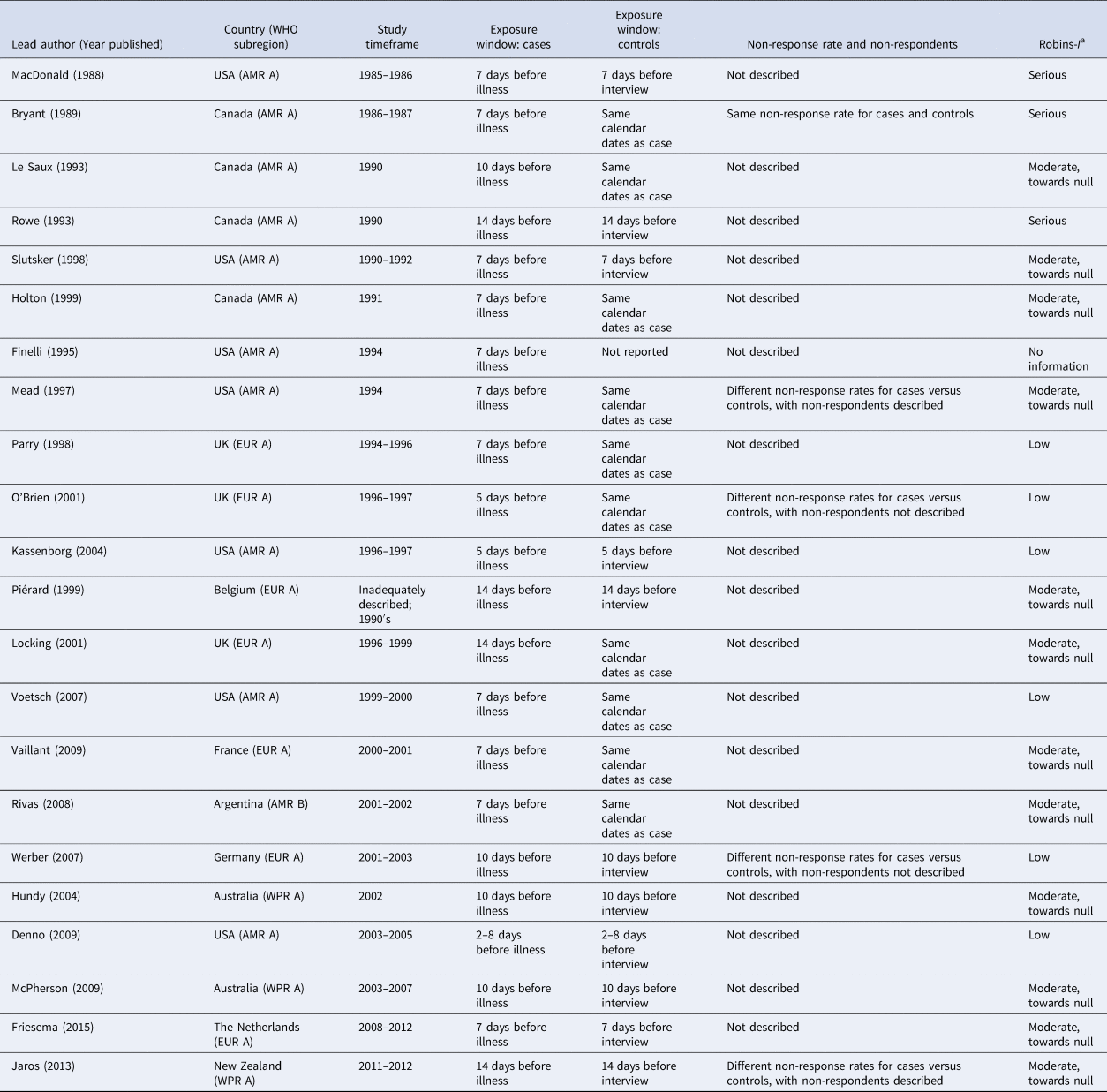
a Modified ROBINS-I categories:
1. low risk of bias in the reported OR's for food exposures.
2. moderate risk of bias in the reported OR's for food exposures, with the bias likely towards the null (i.e. towards an OR = 1).
3. moderate risk of bias in the reported OR's for food exposures, with the bias likely away from the null (i.e. away from an OR = 1).
4. serious risk of bias (either toward or away from the null): the study has some important problems.
5. critical risk of bias (either toward or away from the null): the study is too problematic to provide useful evidence.
6. no information.
In considering all risk of bias assessment items together, two of the 22 studies (9%) were not considered reliable, taking study limitations into consideration [Reference Rowe22,Reference Finelli25]. Of the 22 studies, six (27%) were assessed to have a low risk of bias in the reported ORs for food exposures; 12 (55%) were assessed to have a moderate risk of bias in the reported ORs for food exposures, with the bias likely towards the null (i.e. towards an OR = 1); three (14%) were assessed to have a serious risk of bias (either toward or away from the null); and one did not have adequate information to make an assessment (Table 2).
Of the 22 studies, 18 (82%; Table 1) included individuals of all ages, with 15 providing results for all age groups combined in their estimates, two providing results stratified by age [Reference Werber11,Reference Friesema38] and one providing results for both all participants combined and for the subset of children [Reference Piérard30]. Of the 22 studies, four (18%) included only children. Most studies (16/22; 73%) included cases and controls drawn from the general population, with cases identified either via existing laboratory surveillance with public health notification (10/16; 63%) or via active case ascertainment at the laboratory level (6/16; 38%) and controls identified via random/semi-random sampling of the general population (including via existing registries, control databases, or random digit dialing; 8/16; 50%), or via the same facility or practice (5/16; 31%) or the same neighbourhood as the case (2/16; 13%). The remaining six of the 22 studies (27%) drew cases from specific facilities, via active case ascertainment within laboratories (3/6; 50%), emergency rooms (1/6; 17%) and by physicians (1/6; 17%), or health record reviews (1/6; 17%). These six studies selected controls from the same facility as the cases (4/6), as well as from the case's friends (1/6) and neighbourhood (1/6). In 20 of the 22 studies (91%), cases were defined as symptomatic individuals with laboratory confirmation of STEC. In one study, cases were defined as those with post-diarrhoeal hemolytic-uremic syndrome, of whom 82.4% had a laboratory-confirmed STEC infection [Reference Rowe22]. In another study, cases were either symptomatic individuals with laboratory confirmation of STEC or those with post-diarrhoeal hemolytic-uremic syndrome [Reference Rivas34].
Food items associated with STEC infection
Extractable information on the relative odds of exposure to a given food, for cases as compared to controls, was provided by all but one paper [Reference Denno36]. Thus, we extracted data from 21 papers, for 245 individual measures in 11 food categories and across three status types: raw or undercooked, not raw (i.e. adequately cooked, treated, pasteurized, or other mechanism) and unknown (Table 3). Of the 245 individual measures extracted, 237 provided useable data (Table 4). Within the dairy category, the food items could not be divided by animal source because this information was only available for two items from one study (ewes' milk cheese and goats' milk cheese [Reference Vaillant33]). Similarly, the animal source was not provided for ‘eggs’, which were reported in two studies [Reference Locking31,Reference McPherson37]. The 62 items classified as ‘meat – unspecified’ included items (of which 60 had useable data) that could not be assigned to their animal origin (e.g., beef, pork; Table 3). Of the 38 items classified as ‘produce’ only 11 were reported as specific fruits or vegetables (Table 3). Because there were very few results per specific produce item, this category was not divided further.
Table 3. Categories of the 245 food items extracted from the 21 case-control studies of non-outbreak (i.e. sporadic) STEC infection in humans, ranked in descending order by the number of food items per category
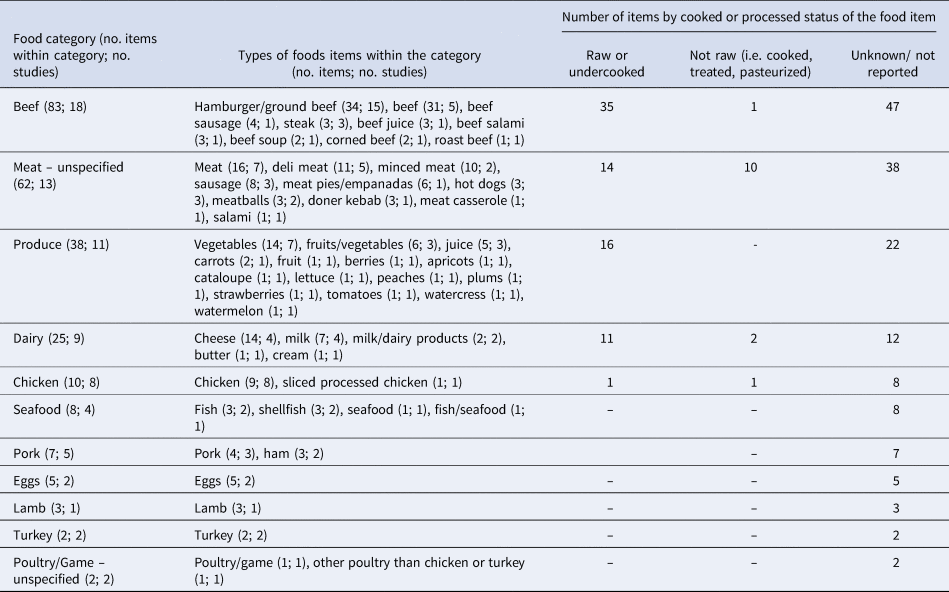
Table 4. Results of the meta-analysis, showing pooled univariate odds ratios (ORs) per food category (significant values shown in bold), ranked in descending order by the number of food items in the category
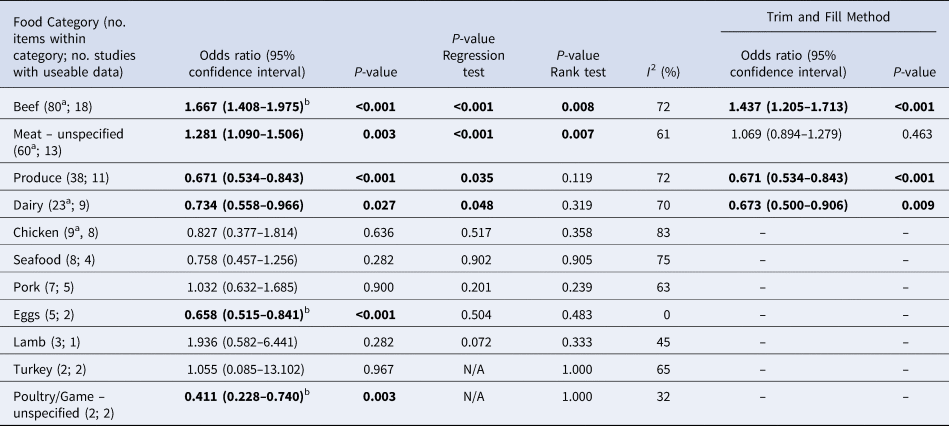
a These numbers are less than in Table 3 because some food items as reported did not have sufficient useable data
b These items remained significant when clustering by study was accounted for
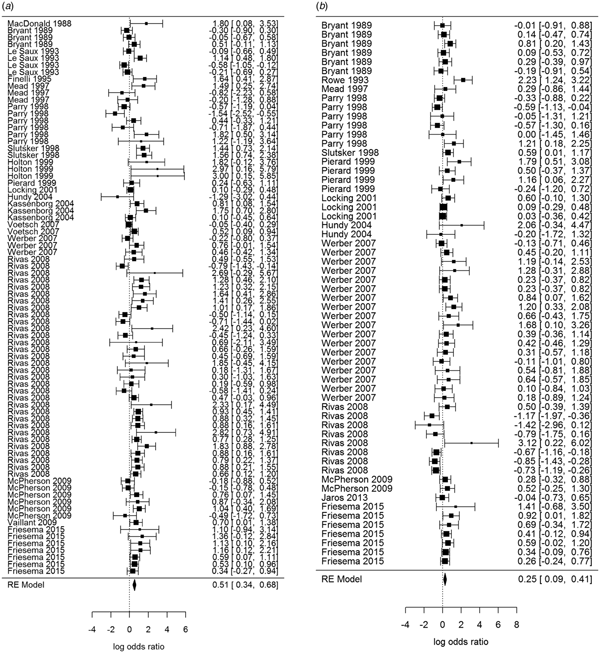
Overall, beef and meat – unspecified were most significantly associated with sporadic STEC infection, although meat-unspecified became non-significant when the trim and fill method was used (Table 4; Fig. 3a and 3b). Produce, dairy, eggs and poultry/game-unspecified were also significant but had ORs less than one. When the alternate approach (see methods) to determining the ORs for each study/food was applied, estimates of the summary ORs neither changed in magnitude, nor direction, nor significance (Supplementary Materials, Section B). Under the sensitivity analysis, which accounted for clustering by study, only beef, eggs and poultry/game-unspecified remained significant.
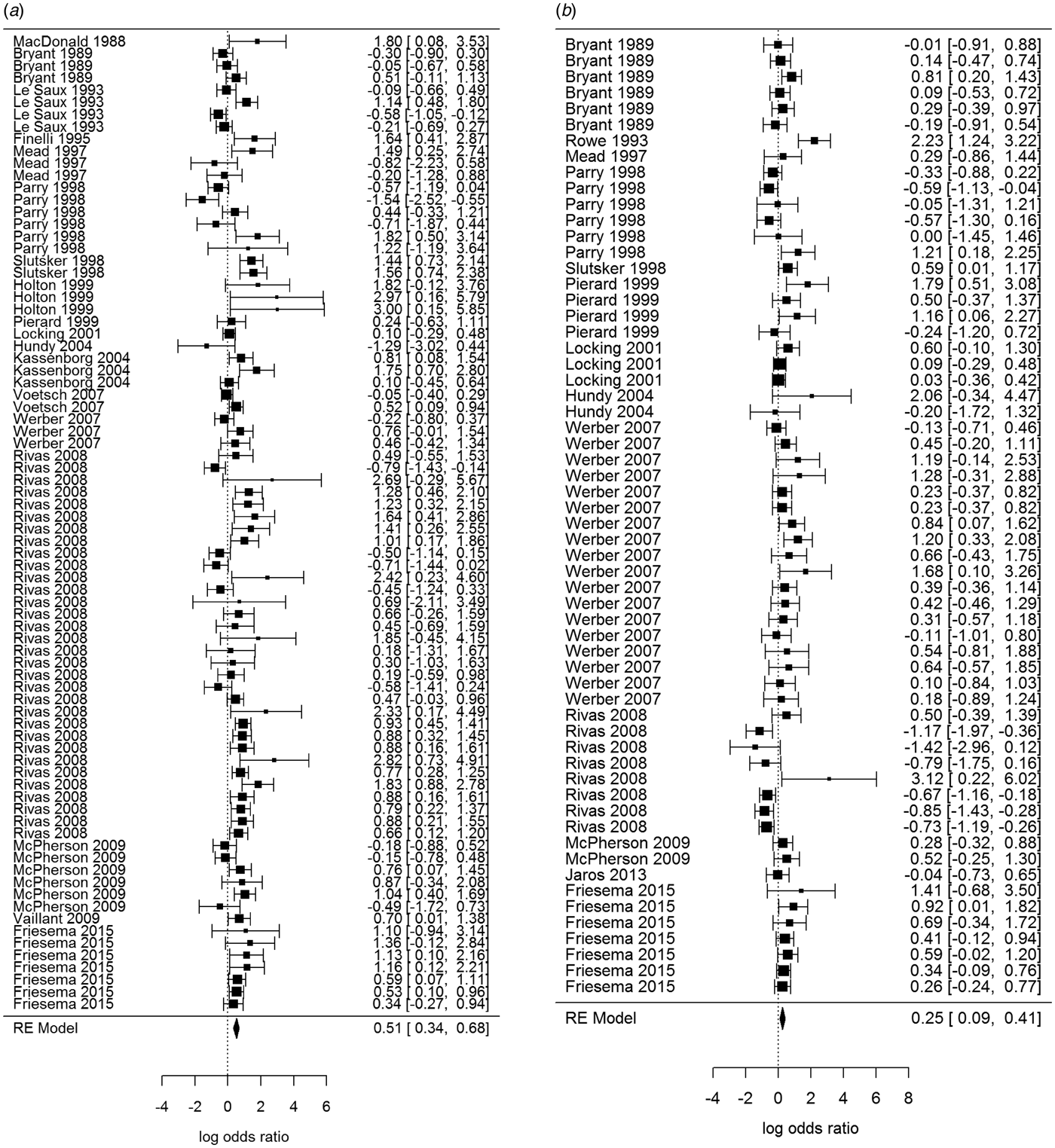
Fig. 3. Forest plots of the log odds ratio (OR) of the risk of human STEC infection from beef (a) and meat-unspecified (b), showing the overall pooled OR together with the 95% confidence interval (CI); ordered from oldest (top) to newest (bottom) study.
Significant food categories varied moderately by WHO subregion (Table 5). In the Americas A region, beef and meat – unspecified remained the significant risk factors for STEC, whereas in the Americas B and European A regions, the only significant risk factor was beef and in Western Pacific A region the only significant risk factor was chicken. Under the alternate approach to determining the ORs for each study/food, our estimates of the summary ORs by WHO subregion changed: in the Americas A region beef became non-significant and in European A region meat-unspecified became significant (Supplementary Materials, Section B). Under the sensitivity analysis, beef remained the significant risk factor for STEC in the Americas A and B region and chicken in the Western Pacific A region; no factors remained significant risks in the European A region.
Table 5. Results of the meta-analysis for each World Health Organization (WHO) Sub- Region, showing pooled univariate odds ratios (ORs) per food category (significant values shown in bold)
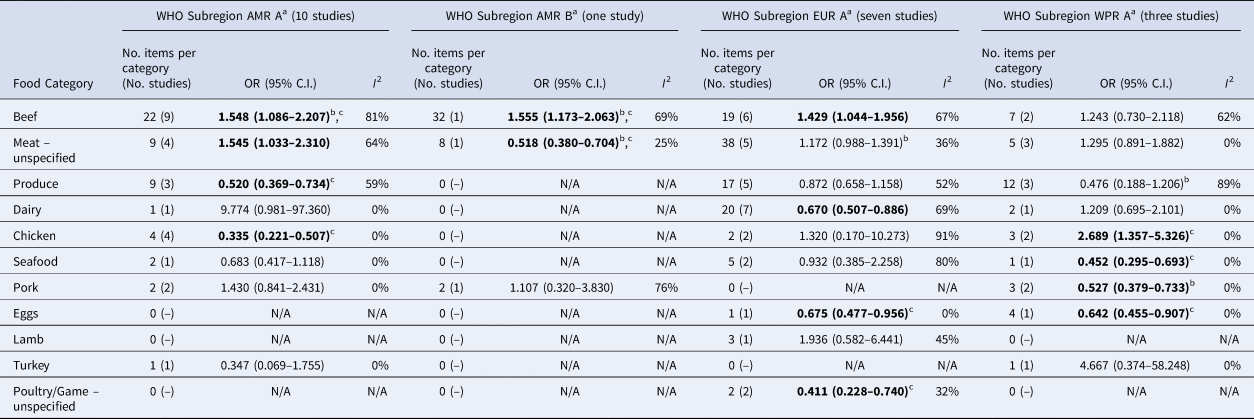
a WHO Subregions comprise the following countries:
Region of the Americas A (AMR A): Canada, Cuba, USA.
Region of the Americas B (AMR B): Antigua and Barbuda, Argentina, Bahamas, Barbados, Belize, Brazil, Chile, Colombia, Costa Rica, Dominica, Dominican Republic, El Salvador, Grenada, Guyana, Honduras, Jamaica, Mexico, Panama, Paraguay, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago, Uruguay, Venezuela (Bolivarian Republic of).
European Region A (EUR A): Andorra, Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Monaco, Netherlands, Norway, Portugal, San Marino, Slovenia, Spain, Sweden, Switzerland, United Kingdom of Great Britain and Northern Ireland.
Western Pacific Region A (WPR A): Australia, Brunei Darussalam, Japan, New Zealand, Singapore.
b Using trim and fill method.
c These items remained significant when clustering by the study was accounted for.
Our exploratory meta-regression analysis of the association between different study characteristics and ORs for food categories is shown in Table 6. Study population age was significant for dairy, as studies in children yielded lower ORs than studies of all ages. Study subregion was significant for meat – unspecified (with the study from the Americas B region yielding lower ORs than studies from the Americas A region), produce (with studies from the European A region yielding higher ORs than studies from the Americas A region), dairy (with studies from the European A region yielding lower ORs than studies from the Americas A region) and chicken (with studies from the European A and Western Pacific A regions yielding higher ORs than studies from the Americas A region; data not shown). One of the two measures of risk of bias was significant for meat – unspecified, dairy and chicken, as follows. For both meat – unspecified and dairy, the study whose findings were considered not reliable yielded higher ORs than studies whose findings were considered believeable (whereas findings did not differ significantly by Robin's I). For chicken, studies with serious risk of bias, either toward or away from the null, yielded lower ORs than studies with a low risk of bias (whereas findings did not differ signifcantly by overall study believability; data not shown). Publication year and whether the food item was raw/undercooked, not raw, or unknown were not significant moderating factors. Results under the alternate approach varied slightly and are given in the Supplementary Materials (Section C). Under the sensitivitiy analysis, even fewer significant modifiers remained.
Table 6. Results from the meta-regression analysis of the univariate moderating effects of study characteristics, by food category, with significant values shown in bold (food categories with <20 results are not shown)

a These items remained significant when clustering by the study was accounted for.
Discussion
In this study, our aim was to determine the relative importance of different foods for sporadic, foodborne illnesses caused by STEC. Here, we found that beef was the most significant food item risk factor for STEC illness, significant in the Americas and Europe but not in the Western Pacific region, where chicken was the most significant food item risk factor. These findings for beef and chicken were not significantly moderated by the raw or cooked status of the food item, nor the publication year of the study. Note that these findings describe the importance of different food items relative to other foods and cannot be used to infer the importance of food exposures compared to non-food exposures (e.g. environmental, animal, person-to-person exposures). Additionally, because we did not identify any case-control studies from the African, South-East Asian, nor Eastern Mediterranian subregions, the relevance of these results for these regions is unclear. Future investigation to determine the burden of foodborne STEC in these regions is needed.
As expected, this study corroborates the existing body of evidence that beef is a major source of exposure to STEC, thereby reinforcing the need for interventions in this commodity. Chicken was also identified as a significant food source, specifically in the Western Pacific A subregion. This finding differs from that of a recent global expert elicitation study [Reference Hoffman42], which ranked beef as the top food source of STEC infection in this subregion. However, it is important to note that the expert elicitation did not ask explicitly about poultry as a source of STEC (instead asking explicitly about beef, small ruminants' and pigs' meat, dairy, vegetables and fruits and nuts and including an ‘other’ category within which additional foods could be identified). The discrepancy between our results and those from the expert elicitation may relate to the types of foods explicitly investigated by case-control vs. expert elicitation studies. Another reason may be that the contributors to the expert elicidation study were identifying food exposures for all STEC infections, both outbreak and sporadic and large outbreaks of STEC associated with chicken have rarely been reported, though STEC, including STEC O157, have been isolated from chickens and their meat [Reference Persad, Lejeune, Sperandio and Hovde43, Reference Ferens and Hovde44]. To guide policy and prevention decisions, methods are needed that allow different types of evidence about sources of foodborne infections (e.g. from meta-analyses of case-control studies, outbreak analyses, expert elicitations, risk assessments) to be synthesised. Finally, chicken as a significant food source in the Western Pacific Region A could be an artifact of the analysis; however, the possibility that it is a significant source of exposure in this region cannot be discounted, as regional differences in significance could arise from differences in consumption patterns or preferred preparation methods of the commodity.
Here, produce, dairy, eggs, poultry/game – unspecified were also significant but had ORs less than one. For the purpose of source attribution, we do not draw conclusions on factors associated with a statistically significant reduced risk of disease, in part because of the impact of bias inherent in individual case-control studies and thus to the final meta-analysis. While this is true for all exposures and all data that originate from interviews with patients and controls, it is particularly important when making inferences on the protective effect of specific exposures, which may eventually also be routes for infection [Reference Domingues16,Reference Domingues17]. Thus, any ORs less than one are reported herein but not interpreted further.
The way in which sporadic case-control studies are conducted and reported influences the type of data available for analyses such as this one. First, although case-control questionnaires of sporadic enteric illnesses like STEC often include an extensive list of potential risk factors, such as a list of food items, the specific food items included are often those for which there is an established or suspected association with illness, so as not to overburden study participants. Second, of the extensive list of food items for which data are collected, many may not have results reported in the final publication, or they may be reported in aggregate. For example, in one study, the questionnaire included 16 questions about different types of meats consumed, while the paper reported numerical results in aggregate as ‘eating meat’ [Reference Jaros39].
Finally, publication culture prioritises reporting significant over non-significant results. Here, we noticed that significant findings were more often reported with extractable numbers, whereas non-significant findings were often reported as text without numeric values (e.g. ‘eating ground beef was not associated with infection in this study’ [Reference O'Brien, Adak and Gilham28]), so we could not include these findings in our analyses. We attempted to overcome the limited availability of raw data by back-calculating raw values from ORs and SEs. Nevertheless, our results are likely skewed towards those food items for which there was established evidence or a strong hypothesis of risk at the time of the study, as well as results for which there were statistically significant findings. Thus, we recommend that primary research authors follow standard reporting guidelines for observational studies [Reference von Elm45] and that they make the questionnaires and raw data available (e.g. supplementary materials, data repositories). Some of the more recent studies included here did provide questionnaires and summary data as supplementary materials [Reference Jaros39e.g. ]; we commend this comprehensive reporting while reiterating the need for studies to follow standard reporting guidelines and make publically accessible their questionnaires and raw data.
There were only 237 individual measures resulting from 21 unique studies included in the analysis and the majority of data were for beef and for the Americas A region, indicating even more sparse data for other food groups and regions. Indeed, our results from the Americas B region come from one study in a single country, conducted in children 0–15 years old, suggesting that the results for this subregion, in particular, be interpreted with caution. Additionally, there was temporal variation in study periods by region: in the Americas, studies were predominantly conducted in the mid-1980s and 1990s; in Europe, the early 1990s to early 2000s and in the Western Pacific region, the 2000s to 2012. Our findings are thus subject to limitations common to such meta-analyses based on sparse data and it is possible that the subregion effect seen here may actually be due to changes in risks over time. We explored publication bias using common methods, however, these tests are typically only reliable if there are 10 or more studies and their applicability to observational studies is unclear due to the presence of other biases (e.g. confounding) [Reference Sterne46]. Therefore, significant publication bias tests identified in this review may be due to study confounding or other biases rather than possible publication bias. There are also some limitations to the meta-regression analyses used to explore the potential effects of study characteristics on our estimates. For example, the exploration of significant covariates through meta-regression becomes increasingly less powerful as sample sizes (i.e. the number of studies) decrease and false positive findings are possible with multiple covariates assessed across numerous outcome groups [Reference Thompson and Higgins47]. Finally, we identified a potential effect of study bias on the results for meat – unspecified, dairy and chicken and thus they should be interpreted with caution. The results for beef, on the other hand, were not found to be affected by study bias and thus we have higher confidence in these findings.
Despite these limitations, our findings corroborate that interventions aimed at reducing the transmission of STEC via beef and other foods of animal origin, are important priorities in order to reduce the burden of foodborne STEC illness in the population, with additional studies needed to determine if this recommendation holds true for regions outside the Americas and Europe.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268819001183.
Author ORCIDs
B. Devleesschauwer, 0000-0002-2867-6892; S. M. Pires, 0000-0002-7751-1509; I Young 0000-0002-5575-5174; S. E. Majowicz, 0000-0002-0006-8369.
Acknowledgements
The authors thank the Joint Food and Agriculture Organization/World Health Organization Core Expert Group on STEC/VTEC (see: http://www.fao.org/3/ca0032en/CA0032EN.pdf for list of members) for consulting on the comprehensive list of STEC serogroups we used as search terms and Librarian Jackie Stapleton (University of Waterloo) for assistance designing the literature search. The authors also thank Lapo Mughini-Gras (RIVM), Lan XU (Belgian Cancer Centre/Unit of Cancer Epidemiology, Sciensano, Belgium) and Martina Otavova (Sciensano, Belgium) for screening non-English articles in various languages and Patricia Griffin and Weidong Gu (Centers for Disease Control and Prevention, USA) for comments on earlier drafts of this work.
Conflict of interest
SEM, SMP and BD have worked previously with the World Health Organization under the Foodborne Disease Burden Epidemiology Reference Group. SEM is an Associate Editor at Epidemiology and Infection, and has served as a paid Expert on behalf of the Attorney General of Canada in legal proceedings, providing evidence on the public health risks and benefits of unpasteurized milk. No other authors report any conflicts of interest.
Disclaimer
This study was commissioned and paid for by the World Health Organization (WHO; contract with S. Majowicz). Copyright in the original work on which this article is based belongs to WHO. The authors have been given permission to publish this article. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the views, decisions, or policies of the WHO.