Angiosperms are unequivocally the most important plant group for human beings and current ecosystems. They are commonly characterised by their unique reproductive organs; i.e., flowers. In spite of all the effort invested in flowers, where they came from has long been a puzzle for botanists. Arber and Parkin related the female part of Bennettitales with the gynoecium of Magnolia and declared the latter as the most primitive angiosperm (Arber & Parkin Reference Arber and Parkin1907). Almost all plant systematicists have been heavily influenced by this hypothesis. The morphology and anatomy of the female parts of Bennettitales, in which the ovules, with exserted micropylar tubes, are surrounded by interseminal scales, were well-documented in the last century (Wieland Reference Wieland1906; Seward Reference Seward1919; Rothwell & Stockey Reference Rothwell and Stockey2002, Reference Rothwell, Stockey and Gee2010; Stockey & Rothwell Reference Stockey and Rothwell2003; Crane & Herendeen Reference Crane and Herendeen2009). Ironically, the more that is learned of Bennettitales, the greater the gap between Bennettitales and angiosperms appears to be. A magnoliaceous carpel was thought to be derived from a foliar part bearing ovules along its margins (Eames Reference Eames1961; Cronquist Reference Cronquist1988; Dilcher Reference Dilcher and Gee2010). However, relating the female part of Bennettitales to conduplicate magnoliaceous carpels is now problematic because evidence favouring this hypothesis has been lacking. Conversely, evidence negating this hypothesis is continuously emerging (Liu et al. Reference Liu, Hilu and Wang2014; Miao et al. Reference Miao, Liu, Wang and Wang2017; Zhang et al. Reference Zhang, Liu and Wang2017).
Numerous plants and insects have been found in the Daohugou region (Zheng et al. Reference Zheng, Zhang and Gong2003; Ji et al. Reference Ji, Chen, Wang, Jin, Zhang, Liu, Zhang, Yao, Ji, Yuan, Zhang and You2004; Huang et al. Reference Huang, Nel, Shen, Selden and Lin2006; Zhou et al. Reference Zhou, Zheng and Zhang2007; Ren et al. Reference Ren, Labandeira, Santiago-Blay, Rasnitsyn, Shih, Bashkuev, Logan, Hotton and Dilcher2009, Reference Ren, Shih, Gao, Yao and Zhao2010; Wang et al. Reference Wang, Zheng and Jin2010b; Zheng & Wang Reference Zheng and Wang2010; Wang & Zhang Reference Wang and Zhang2011; Peng et al. Reference Peng, Liu, Kuang, Jiang and Xu2012), and there is general consensus on the Middle Jurassic age of the Jiulongshan Formation (formerly Daohugou Formation) (Ren et al. Reference Rothwell and Stockey2002; Zhang Reference Zhang2002, Reference Zhang2006; Shen et al. Reference Shen, Chen and Huang2003; Li et al. Reference Li, Li, Wang, Zheng and Zhang2004; Liu et al. Reference Liu, Liu, Li, Zhang, Zhang, Li and Xia2004; Ji et al. Reference Ji, Liu, Chen, Ji, Lu, You and Yuan2005; Huang et al. Reference Huang, Nel, Shen, Selden and Lin2006, Reference Huang, Zompro and Waller2008a, Reference Huang and Nelb, Reference Huang, Selden and Dunlop2009; Huang & Nel Reference Huang and Nel2007, Reference Huang and Nel2008; Petrulevicius et al. Reference Petrulevicius, Huang and Ren2007; Sha Reference Sha2007; Zhou et al. Reference Zhou, Zheng and Zhang2007; Lin et al. Reference Lin, Huang and Nel2008; Liu & Ren Reference Liu and Ren2008; Selden et al. Reference Selden, Huang and Ren2008; Zhang et al. Reference Zhang, Ren, Pang and Shih2008, Reference Zhang, Li, Yang and Ren2009, Reference Zhang, D'Rozario, Yao, Wu and Wang2011; Chang et al. Reference Chang, Zhang, Renne and Fang2009, Reference Chang, Zhang, Hemming, Mesko and Fang2014; Liang et al. Reference Liang, Vrsansky, Ren and Shih2009; Shih et al. Reference Shih, Liu and Ren2009; Wang et al. Reference Wang and Zhang2009a, Reference Wang and Zhangb, Reference Wang and Zhangc, Reference Wang, Krings and Taylor2010a; Wang & Ren Reference Wang and Ren2009; Wang & Zhang Reference Wang and Zhang2009a, Reference Wang and Zhangb). Ar40/Ar39 and SHRIMP U/Pb datings have been performed on the volcanic rocks in the overlying Tiaojishan Formation (Chen et al. Reference Chen, Ji, Liu, Zhang, Song and Liu2004; Ji et al. Reference Ji, Chen, Wang, Jin, Zhang, Liu, Zhang, Yao, Ji, Yuan, Zhang and You2004), suggesting an age of at least 164Ma for the fossiliferous Jiulongshan Formation. In addition to various animal fossils (especially insects), abundant fossil plants have been reported from this region. According to current literature, the Daohugou flora includes algae of one species (within the Chlorophyceae), four genera and six species of bryophytes (Daohugouthallus, Metzgerites, Muscites, Ningchengia), two genera and two species of Lycopodaceae (Lycopodites, Selaginellites), two genera and two species of sphenophytes (Annularia, Equisetites), four genera and six species of Filicales (Coniopteris, Osmunda, Eboracia, Sphenopteris), seven genera and 12 species of cycads (Pterophyllum, Anomozamites, Nissoniopteris, Williamsonia, Weltrichia, Cycadolepis, Tyrmia), four genera and four species of Czekanowskiales (Czekanowskia, Solenites, Leptostrobus, Ixostrobus), four genera and six species of Ginkgoales (Yimaia, Ginkgoites, Baiera, Sphenobaiera), 13 genera and 20 species of Coniferales (Pityocladus, Pityospermum, Schizolepis, Austrohamia (Yanliaoa), Brachyphyllum, Elatocladus, Amentotaxus, Taxus, Nageiopsis, Podocarpites, Cephalotaxopsis, ?Pseudofrenelopsis, Podozamites), two genera and two species of Caytoniales (Caytonia, Sagenopteris), three genera and three species of seeds/fruits with unknown affinities (Conites, Problematospermum, Carpolithus), and three genera and three species of angiosperms (Solaranthus, Juraherba, Yuhania) (Zheng et al. Reference Zheng, Zhang and Gong2003; Li et al. Reference Li, Li, Wang, Zheng and Zhang2004; Zhou et al. Reference Zhou, Zheng and Zhang2007; Wang et al. Reference Wang, Krings and Taylor2010a, Reference Wang, Zheng and Jinb; Zheng and Wang Reference Zheng and Wang2010; Pott et al. Reference Pott, McLoughlin, Wu and Friis2012; Heinrichs et al. Reference Heinrichs, Wang, Ignatov and Krings2014; Dong et al. Reference Dong, Yang, Zhou and Huang2016; Han et al. Reference Han, Liu, Liu, Mao, Jacques and Wang2016; Liu & Wang Reference Liu and Wang2016b). As reported here, these works converge on a Middle Jurassic age for Zhangwuia.
Recently, a bennettitalean plant, Foxeoidea, was reported with its ovules surrounded by interseminal scales, although its ovules were not fully enclosed as they are in angiosperms (Rothwell & Stockey Reference Rothwell, Stockey and Gee2010). To help bridge this gap between Foxeoidea and typical angiosperms, we here report a fossil reproductive organ, Zhangwuia gen. nov., from the Jiulongshan Formation of the Middle Jurassic (>164Ma) of Daohugou Village, Inner Mongolia, China [119°15′E, 41°19′N]. Zhangwuia demonstrates a great resemblance to angiosperm flowers, including its flower-like organisation, surrounding foliar parts, and, most importantly, angio-ovuly in the female part. Interestingly, the general morphology of Zhangwuia demonstrates a resemblance to Bennettitales. Such a mosaic combination of characters implies that at least some Bennettitales have the potential to reach angio-ovuly. In this way, Zhangwuia could narrow the evolutionary gap between angiosperms and gymnosperms.
1. Material and methods
The fossil material was collected by a local fossil collector, Mr Hongtao Cai, from the outcrop of the Jiulongshan Formation near Daohugou Village, Ningcheng, Liaoning, China (119.236727°E, 41.315756°N; Fig. 1), and it was donated to the Nanjing Institute of Geology and Paleontology, Chinese Academy of Sciences (CAS).
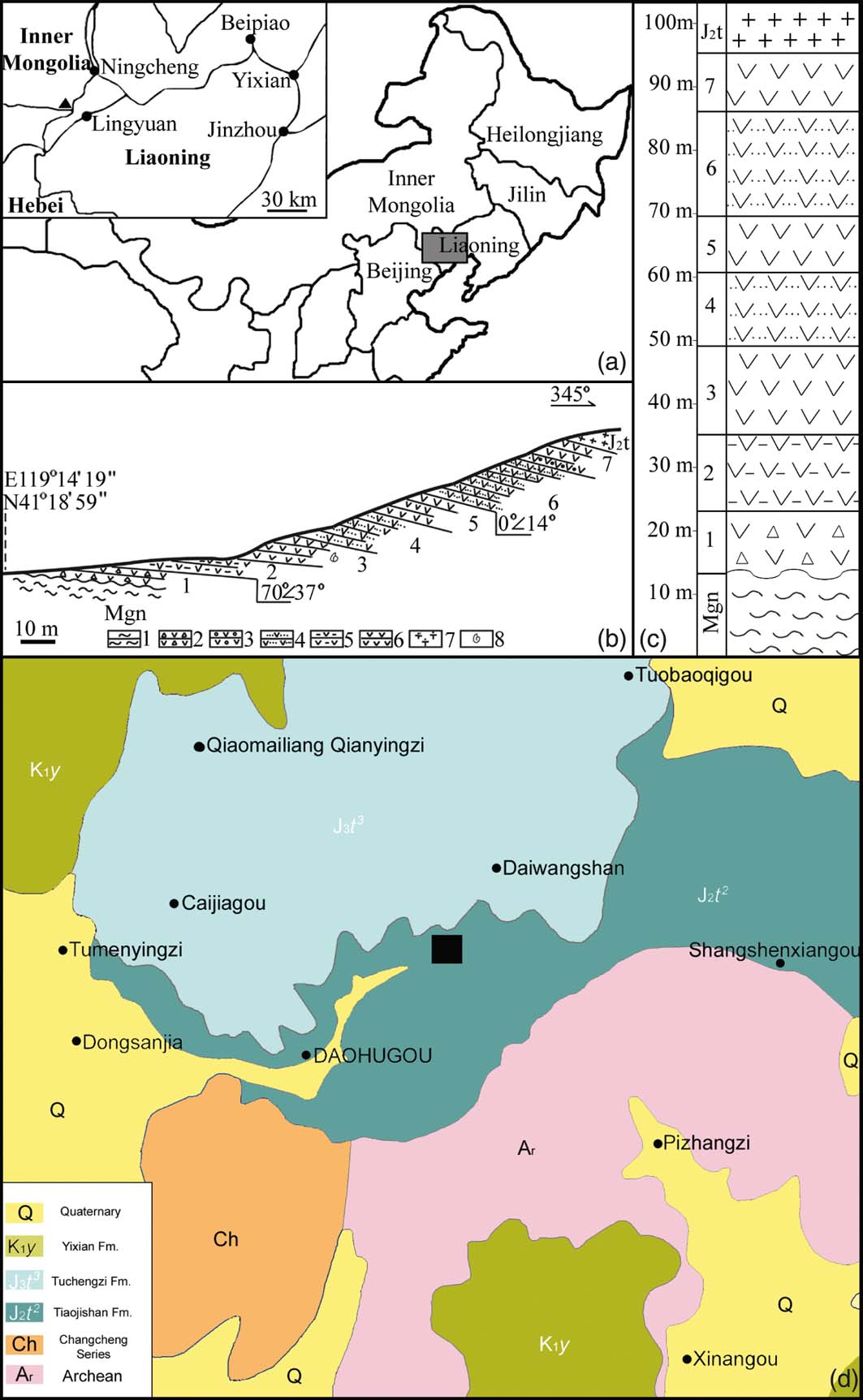
Figure 1 Geological information for the Jiulongshan Formation at Daohugou Village. Modified from Tan & Ren (Reference Tan and Ren2009): (a) geographical position of the fossil locality, Daohugou Village, Ningcheng, Inner Mongolia, China. The rectangular region is shown in detail in the inset, in which the black triangle represents Daohugou Village and the black dots represent cities in the region. (b) Geological section of the Jiulongshan Formation near Daohugou Village. Layer 3 is the major fossil-yielding layer: 1 = gneiss; 2 = tuffaceous grand conglomerate; 3 = tuffaceous conglomerate; 4 = tuffaceous siltstone; 5 = tuffaceous mudstone; 6 = tuffaceous shale; 7 = volcanic breccia; 8 = fossil locality. (c) Stratigraphic column of the Jiulongshan Formation near Daohugou Village. Layer 3 is the major fossil-yielding layer. (d) Geological map of Daohugou Village and adjacent region. Rectangle represents the fossil locality.
The general morphology and details of Zhangwuia were observed and photographed using a Nikon SMZ1500 stereomicroscope with a digital camera. More details were further observed and recorded using a Leo 1530 VP scanning electron microscope (SEM) at the Nanjing Institute of Geology and Palaeontology (NIGPAS), Nanjing, China. Micro-computed tomography (CT) observation was performed using 225kv micro-CT (developed by the Institute of High Energy Physics, CAS) at the Key Laboratory of Vertebrate Evolution and Human Origin of CAS in the Institute of Vertebrate Palaeontology and Palaeoanthropology, scanning under a cone-beam energy of 130kV and a flux of 100μA with an 8.8μm slice distance. The transmission images of 1536 slices were reconstructed with a 2048×2048 matrix and 8.8μm pixel size through a 3D image processing software developed by the Institute of High Energy Physics, CAS. The images were processed using VGStudio Max2.2. The final results were saved as images or videos. All photographs were organised together for publication using Photoshop 7.0.
To make our description more neutral, we use the terms ‘female part' and ‘female unit', instead of ‘gynoecium' and ‘carpel' (which are restricted to angiosperms) to describe the morphology of Zhangwuia.
2. Results
Order Incertae sedis
Family Incertae sedis
Genus Zhangwuia gen. nov.
Generic diagnosis. Reproductive organ of radial symmetry. Outer foliar parts inflated, more or less rounded, probably seven in number, radially arranged. Inner foliar parts above the outer foliar parts, radially arranged, in three cycles, approximately seven per cycle, alternate, tongue-shaped, obtuse-tipped, some with a weak midrib. Female part includes numerous spirally arranged female units. Female unit with secluded space, a blunt tip, and an ovule/seed inside.
Type species. Zhangwuia mira gen. et sp. nov.
Etymology. Zhangwu – for the senior Chinese palaeobotanist, Professor Wu Zhang (8 August 1937–27 June 2016).
Horizon. The Jiulongshan Formation, Middle Jurassic.
Age. The Callovian, Middle Jurassic (>164Ma).
Locality. Daohugou Village, Ningcheng, Inner Mongolia, China [119°15′E, 41°19′N].
Zhangwuia mira gen. et sp. nov.
(Figs 2–5)

Figure 2 Zhangwuia mira gen. et sp. nov. and its details. Stereomicroscopy. Holotype, PB21675: (a) the organ with radially arranged inner foliar parts surrounding the central female part (white outline). Rectangles show position of enlargements (b–e); (b) partial view of the organ, enlarged from the lower rectangle in (a), showing the border (white line and arrows) between the inner foliar parts and female part, as well as the border (black line) between receptacle and the female units; (c) detailed view of the female part, enlarged from the rectangle in (b), showing several tips (arrows) of spirally arranged female units; (d) three partially overlapping inner foliar parts, enlarged from the upper rectangle in (a) – the left one has an obtuse tip and no midrib, while the right one has a bent tip and a midrib (white arrow), and note a third inner foliar part (black arrow) in the background; (e) a broken female unit (outline) exposing its internal details, enlarged from the small rectangle (a) (see Fig. 5b, c). Scale bars = 5mm (a); 1mm (b–c); 0.5mm (e).
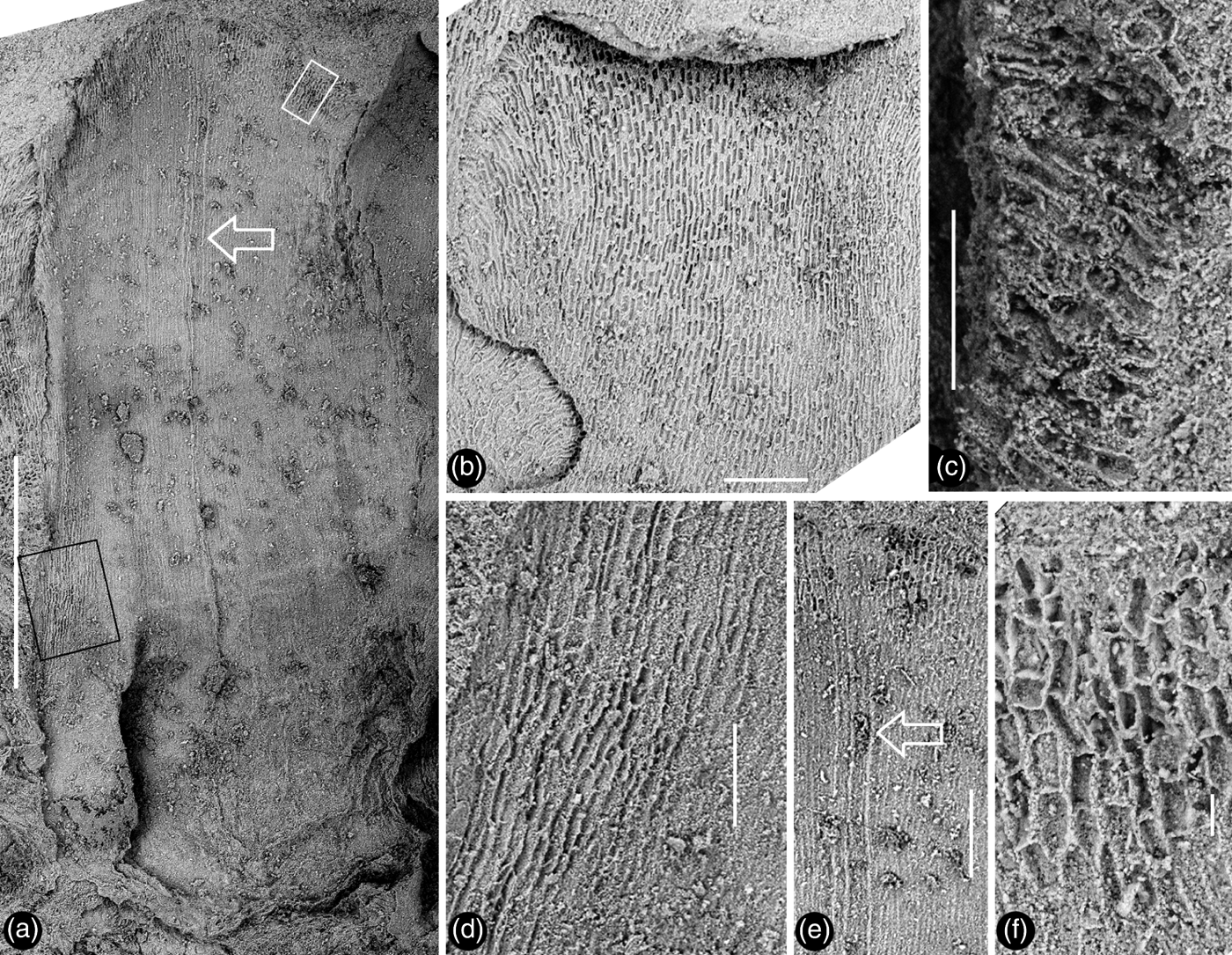
Figure 3 Detailed views of the inner foliar parts of Zhangwuia mira gen. et sp. nov. SEM: (a) an inner foliar part with a midrib (arrow) and an obtuse tip, the no. 1 arrowed part in Fig. 2a; (b) an inner foliar part with a bent tip (top) and no midrib, the no. 4 arrowed part in Fig. 2a; (c) detailed view of a bent tip of an inner foliar part, showing cellular details, from the no. 3 arrowed part in Fig. 2a; (d) cellular details on the margin of an inner foliar part, enlarged from the black rectangle in Fig. 3a; (e) detailed view of the middle portion of the inner foliar part (arrow in Fig. 3a), showing the midrib (arrow); (f) cellular details of the distal portion of the inner foliar part, enlarged from the white rectangle in Fig. 3a. Scale bars = 1mm (a); 0.2mm (b, e); 0.1mm (c, d); 20μm (f).
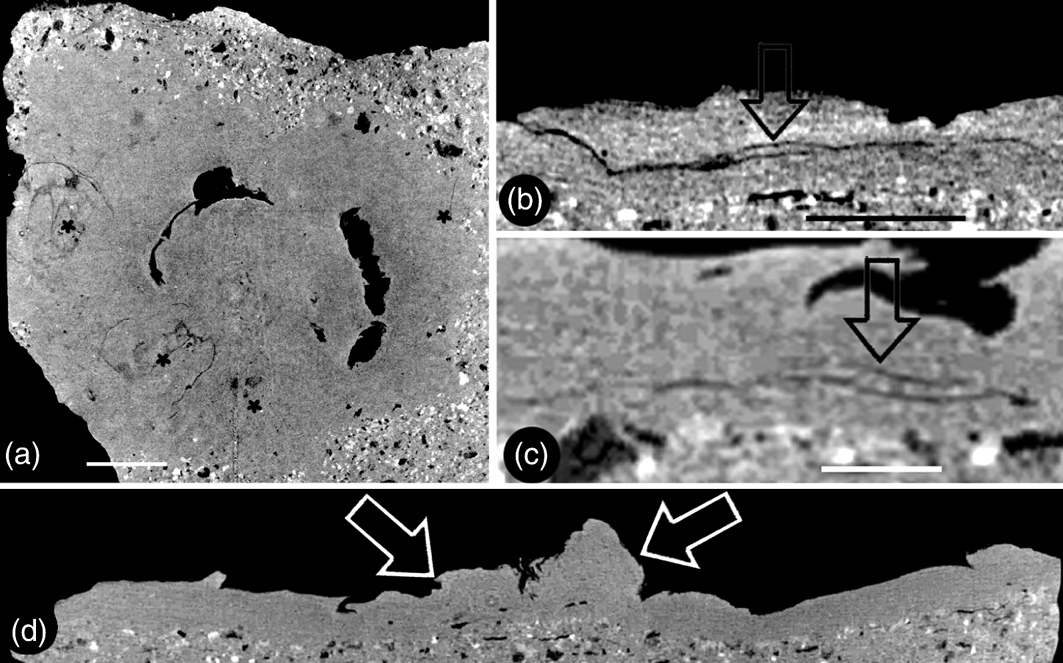
Figure 4 Micro CT virtual sections of Zhangwuia mira gen. et sp. nov.: (a) snapshot of the video showing the organ in cross section. Two of the outer foliar parts are visible in this view. The positions of the outer foliar parts are marked with asterisks. For more information, see supplementary materials, available at https://doi.org/10.1017/S1755691018000257. (b) Tangential section of the organ showing the cross view of an outer foliar part with an inflated portion (arrow) in the middle bottom. (c) Radial section of the organ showing a longitudinal view of the outer foliar part shown in Fig. 4b, with an inflated portion (arrow) near its base. The organ centre is to the right. (d) Longitudinal central section of the organ showing the female unit (left arrow) and the central protruding receptacle (right arrow). Scale bars = 2mm (a); 1mm (b, c).
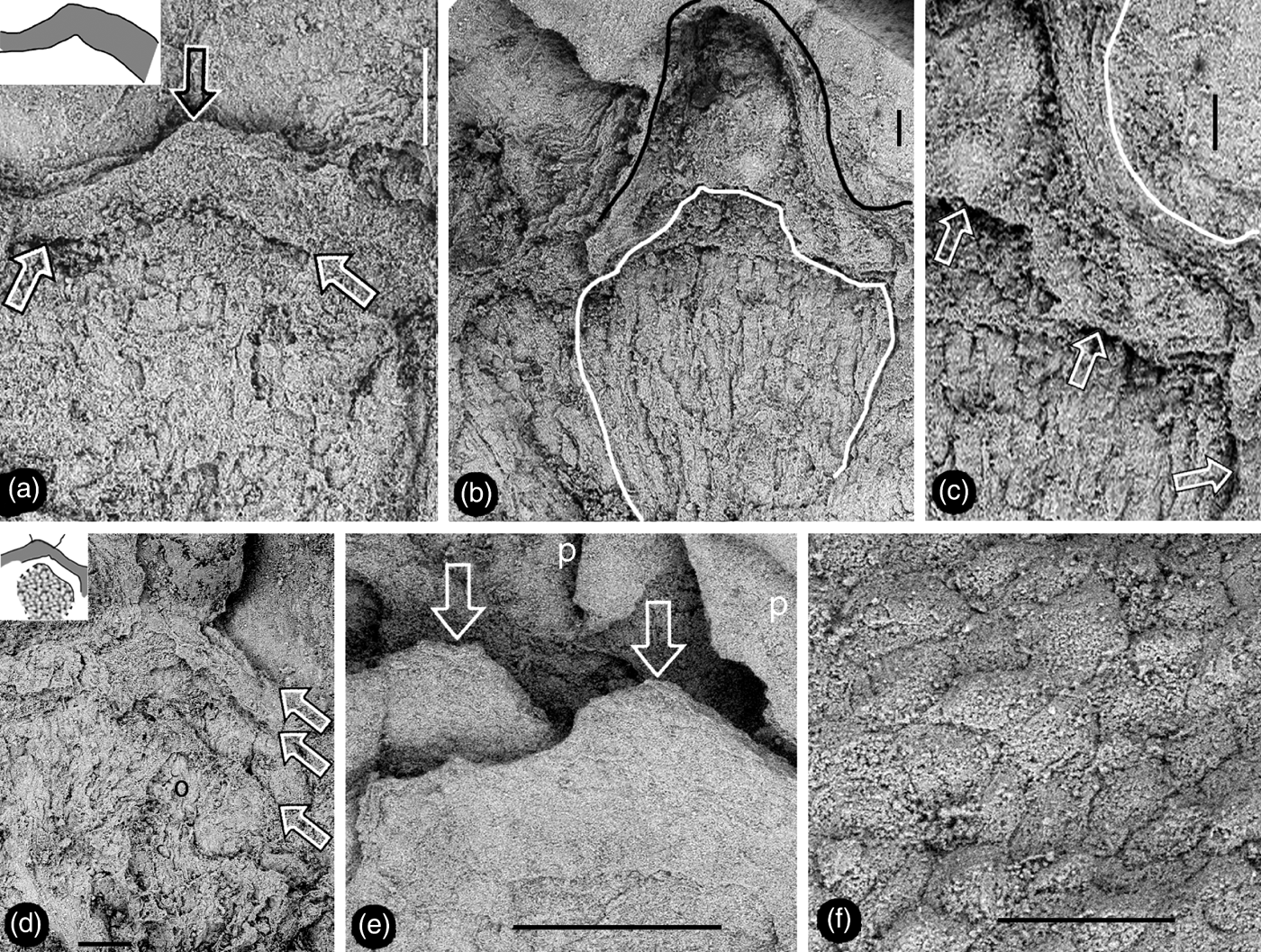
Figure 5 Details of female part of Zhangwuia mira gen. et sp. nov. SEM. (a) Tip (top arrow) of female unit and the margin (lower arrows) of its locule. Inset shows the outline of the locule wall. (b) Details of the female unit shown in Fig. 2e. Note the outline of the female unit (black line) and margin (white line) of the locule. (c) Surface (white line) of the female unit and margin (arrows) of the locule, enlarged from Fig. 5b. (d) An ovule (o) partially covered by other tissue, and a locule wall (between two upper arrows). Note the gap between the ovule and locule wall (between two lower arrows). Refer to the inset. (e) Tips (arrows) of two spirally arranged female units with integral surface. Refer to Fig. 2c. (f) Epidermal cellular details on the tip of a female unit. Scale bars = 0.2mm (a, d); 0.1mm (b, c, f); 0.5mm (e).
Specific diagnosis. (In addition to that of the genus) Organ at least 12mm in diameter. Outer foliar parts 4.3mm long and 3.4mm wide. Inner foliar parts approximately 4mm long and 1.8mm wide. Female part approximately 6mm in diameter at the base. Female unit approximately 1.2mm long, fused basally with adjacent units.
Description. The fossil is brown in colour, embedded in yellowish tuff, preserved in three dimensions, and the exposed part is approximately 12mm in diameter (Figs 2a, 4a, d; supplementary video V1). The organ includes outer and inner foliar parts and female part (Fig. 2a, b). The outer foliar parts are not visible to the naked eye but can be visualised through the application of micro-CT (Fig. 4a; supplementary video V1). The positions of the individual outer foliar parts imply that there may be seven outer foliar parts (Fig. 4a; supplementary video V1). Each outer foliar part is approximately 4.3mm long and 3.4mm wide, more or less rounded, with an inflated portion in the middle base (Figs 4a–c; supplementary videos V1–V3). The inner foliar parts include approximately 23 members preserved alternately in three cycles (Fig. 2a, d). Each inner foliar part is approximately 4mm long and 1.8mm wide, with straight parallel entire margins and an obtuse tip, attached to the receptacle with its whole base, with a longitudinally oriented epidermal texture (Figs 2a, d, 3a, b, d–f). Some of the inner foliar parts may have weak midribs (Figs 2d, 3a, e) or tips bent adaxially (Figs 2a, d, 3b, c). The cells are approximately 45–50μm long and 7–13μm wide in the lateral regions of the inner foliar parts, and approximately 30–80μm long and 19–24μm wide in the midrib region of the inner foliar parts (Fig. 3d–f). The female part is approximately 6mm in diameter, conically formed, tapering distally, and bearing spirally arranged female units (Figs 2a–c, 4d, 5e; supplementary videos V1–V2). The centre of the female part was replaced with sediment during the fossilisation (Fig. 2a, b). The peripheral tissue of the female part is broken, exposing the details of the female units (Figs 2e, 5a–c). The female unit is up to 1.9mm long and 1.7mm wide, and fused with neighbouring units basally (Figs 2c, e, 5e). The surface of the female units is integral, with epidermal cells 50–80×20–50μm (Figs 2c, 5d, e). There is a secluded locule, separated from the exterior space by a 0.2-mm-thick wall, inside each female unit (Fig. 5a, d). An ovule isolated from the locule wall is seen inside the female unit (Fig. 5d).
Specimen number. PB21675.
Etymology. mira for mirus, meaning ‘wonderful' in Latin.
Depository. The Nanjing Institute of Geology and Palaeontology, Nanjing, China.
3. Discussion
‘Carpel' is a frequently used term in angiosperm morphology. There are at least two usages of this term. First, carpel sensu lato designates any structure that encloses the ovules; namely, the basic unit of angiosperm gynoecium. Second, a carpel sensu stricto designates a foliar part enclosing ovules/seeds. The carpel sensu stricto is hinged with the assumption that a carpel is derived from a megasporophyll, bearing ovules along its margins (Arber & Parkin Reference Arber and Parkin1907). This assumption has constituted the foundation for angiosperm systematics for more than a hundred years. However, a ‘sporophyll' is purely an imaginary part that has never been seen in any fossil or extant plant (Wilson Reference Wilson1937; Melville Reference Melville1963; Miao et al. Reference Miao, Liu, Wang and Wang2017) because all ovules are borne on branches rather than on leaves (Herr Reference Herr1995) and the only leaf-like ‘megasporophyll' seen in Cycas is a result of mechanical pressure from the adjacent ovulates parts during the development (Wang & Luo Reference Wang and Luo2013). Deciphering how the angiospermous carpel can be derived from the bennettitalean female part has been difficult for botanists. Until this question is answered satisfactorily, it is impossible to securely establish any of the hypotheses concerning the relationship between angiosperms and Bennettitales, as well as the systematics of angiosperm. Therefore, bridging the gap between angiosperms and Bennettitales using fossil evidence is of crucial importance for plant systematics.
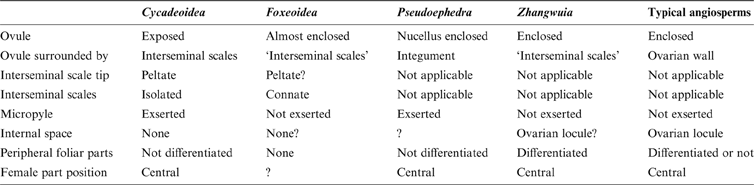
The resemblance between Zhangwuia and Bennettitales is conspicuous. Bennettitales are a fossil group that have been well-documented by various authors in the past century (Wieland Reference Wieland1906; Seward Reference Seward1919; Rothwell & Stockey Reference Rothwell and Stockey2002; Stockey & Rothwell Reference Stockey and Rothwell2003; Crane & Herendeen Reference Crane and Herendeen2009; Rothwell & Stockey Reference Rothwell, Stockey and Gee2010). The inner foliar parts surrounding the female part in Zhangwuia are comparable to the foliar parts surrounding the female part in Bennettitales (Watson & Sincock Reference Watson and Sincock1992). The receptacle of some Bennettitales is also conical in form and may become filled with sediment (Watson & Sincock Reference Watson and Sincock1992), which is very similar to Zhangwuia. The arrangement of the female units around the receptacle in Zhangwuia appears like that of the seeds and interseminal scales arranged around a receptacle in Bennettitales (e.g., Cycadeoidea, Williamsoniella, Williamsonia, and Buttercarpus (Watson & Sincock Reference Watson and Sincock1992; Rothwell & Stockey Reference Rothwell and Stockey2002; Stockey & Rothwell Reference Stockey and Rothwell2003; Crane & Herendeen Reference Crane and Herendeen2009; Rothwell et al. Reference Rothwell, Crepet and Stockey2009)). If this comparison is valid, there seems to be some phylogenetic relationship between Zhangwuia and Bennettitales. The bennettitalean female part would be identical to Zhangwuia if its ovules were completely covered by the adjacent interseminal scales (Fig. 6c, d). Such ovule-enclosure is almost achieved in another fossil taxon, Foxeoidea (Rothwell & Stockey Reference Rothwell, Stockey and Gee2010). The Middle Jurassic age and the morphology of Zhangwuia favour placing Zhangwuia in the Bennettitales. It is worth emphasising that Zhangwuia demonstrates certain features unexpected for any Bennettitales; namely, the ovules in all Bennettitales (including the problematic Foxeoidea of Rothwell & Stockey Reference Rothwell, Stockey and Gee2010) are consistently, more or less, exposed to the exterior, while the ovules of Zhangwuia are inside the female units. An angiosperm flower is typically characterised by a perianth around a gynoecium and/or an androecium (Eames Reference Eames1961). Similar organisation has been seen in both angiosperms and Bennettitales (Martens Reference Martens1971; Watson & Sincock Reference Watson and Sincock1992; Biswas & Johri Reference Biswas and Johri1997). A female cone in Bennettitales has a heterogeneous surface comprising micropyle apices and polygonal interseminal scale heads (Watson & Sincock Reference Watson and Sincock1992; Crane & Herendeen Reference Crane and Herendeen2009; Rothwell et al. Reference Rothwell and Stockey2009), while the surface of the female part of Zhangwuia is homogeneous, smooth, and integral (Figs 2c, 5e, f) with no trace of a micropylar tube. Furthermore, the locule within the female unit and the ovules inside the locule of Zhangwuia (Fig. 5d) are never seen in other Bennettitales (Rothwell et al. Reference Rothwell and Stockey2009; Table 1), in which the ovules have exserted micropyles and are tightly surrounded by the interseminal scales. Therefore, if Zhangwuia were put into Bennettitales, it would appear that at least some Bennettitales have achieved angio-ovuly through the connation of interseminal scales.
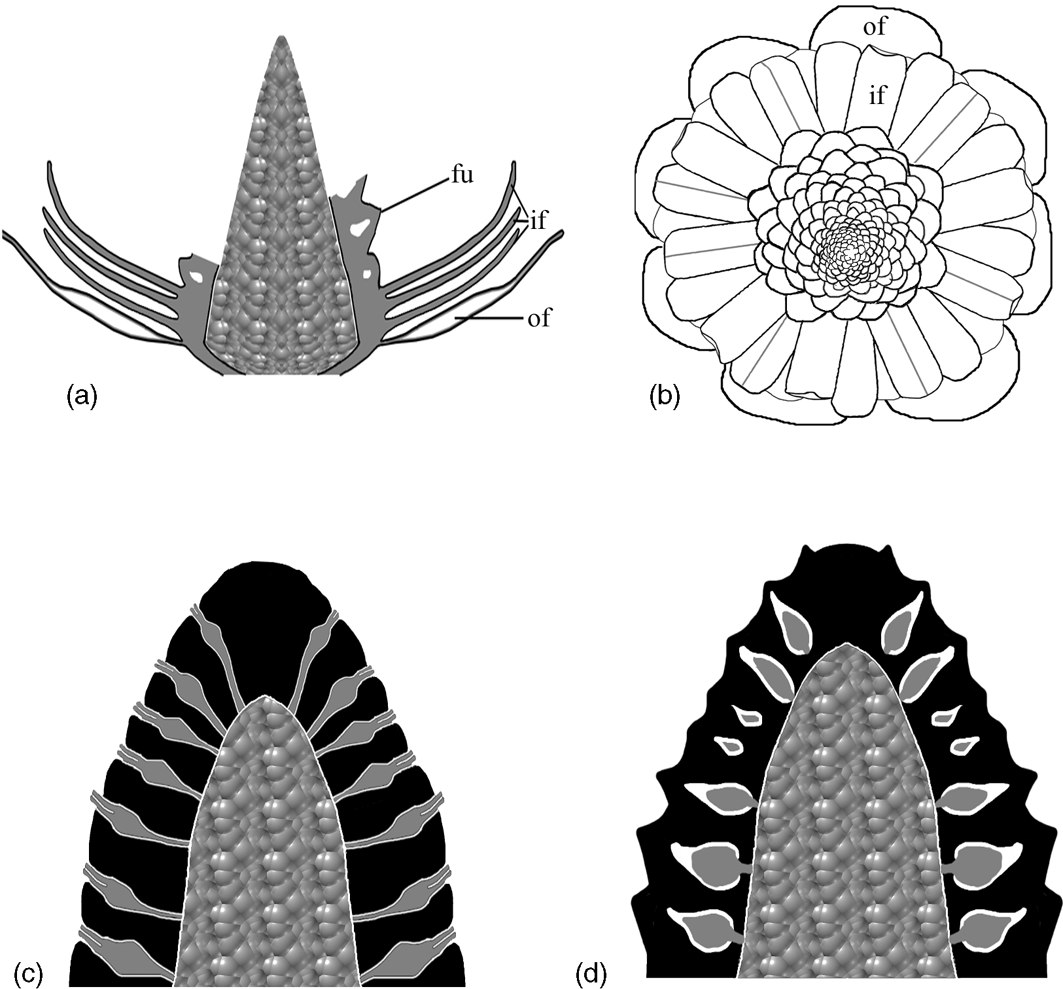
Figure 6 Diagrams showing the structure of Zhangwuia mira gen. et sp. nov. and its comparison with Bennettitales. (a) Vertical profile of the fossil organ, showing outer foliar parts (of), inner foliar parts (if), female units (fu) with internal space, and the receptacle. Note that the female units are missing in the distal portion. (b) Top view of the reconstructed organ, showing outer foliar parts (of), inner foliar parts (if) in three cycles, and female units around the receptacle. Note the presences of midribs and bent tips in some inner foliar parts. (c) Idealised longitudinal section of the female part in Bennettitales. The ovules (grey) have exserted micropylar tubes and are bracketed by interseminal scales (black). Both ovules and interseminal scales are attached to the receptacle. (d) Idealised longitudinal section of the female part in Zhangwuia. The ovules (grey) are separated from the exterior space by the surrounding tissues (black).
Table 1 Comparison among Zhangwuia, some problematic Bennettitales and Gnetales, as well as angiosperms.
It is noteworthy that Zhangwuia bears a resemblance to angiosperm flowers. The arrangement of the female units in Zhangwuia may be compared to female flowers in the inflorescence of Araceae because they both have female units that are crowded on the surface of an axis (Barabé et al. Reference Barabé, Lacroix and Gibernau2003, Reference Barabé, Lacroix, Bruneau, Archambault and Gibernau2004). Because of the breakage, the internal details of the female units of Zhangwuia are observable. As seen in Figs 2e, 5a–d, there are several locules exposed on the broken surface in the female part. These locules are isolated from the exterior space by a wall (Fig. 5a–e), suggesting that a female unit of Zhangwuia has a secluded internal space characteristic of angiosperms. The structure inside the female unit appears to be an ovule (Fig. 5d) as its large size is beyond the scope of microspores, implying the occurrence of angio-ovuly in Zhangwuia. Ovules enclosed before pollination are a feature guaranteeing an angiospermous affinity for the plant in question (Tomlinson & Takaso Reference Tomlinson and Takaso2002; Wang Reference Wang2010). Therefore, Zhangwuia may have a feature that was formerly only restricted to angiosperms.
Such a mosaic combination of characters spanning angiosperms and Bennettitales makes Zhangwuia especially interesting in plant evolution. The enclosure of the ovule as seen in Zhangwuia is not a singular case. For example, Foxeoidea, an unusual element of Bennettitales, has been anatomically documented in the Cretaceous (Rothwell & Stockey Reference Rothwell, Stockey and Gee2010). According to Rothwell & Stockey (Reference Rothwell, Stockey and Gee2010), ovules with micropylar tubes in Foxeoidea are almost completely enclosed by the adjacent interseminal scales that are histologically fused to each other and form a continuous layer around the ovules. The ovule-enclosing in Foxeoidea is quite different from the imagined longitudinal folding of a ‘megasporophyll' bearing ovules along its margins, as suggested by Arber & Parkin (Reference Arber and Parkin1907) and their proponents (Crane Reference Crane1985; Dilcher Reference Dilcher and Gee2010). It should be noted that 1) the ovules in Foxeoidea are not completely enclosed, so Foxeoidea falls well within the scope of Bennettitales; and 2) it is still unclear which parts enclose the ovules in Foxeoidea. According to Rothwell & Stockey (Reference Rothwell, Stockey and Gee2010), the ovules with micropylar tubes in Foxeoidea are surrounded by the adjacent interseminal scales. However, as the researchers admitted, histologically the ‘micropylar tube' is indistinguishable from those of the adjacent interseminal scales because the outer surface of the ‘micropylar tube' is never seen, despite its good anatomical preservation (Rothwell & Stockey Reference Rothwell, Stockey and Gee2010, fig. 4.3b, c). This observation makes an alternative interpretation more likely; namely, that their ‘micropylar tube' is non-existent, and that the ovule-enclosure is completed by the adjacent interseminal scales. If this is the case, then the ovule-enclosing parts of Foxeoidea will be very similar to Zhangwuia in nature. The near-complete enclosure in Foxeoidea can be taken as a precursor to the complete ovule-enclosure of Zhangwuia. In both cases, the ovule-enclosure is completed by the same structure – the former interseminal scales. The anachronism created by Jurassic Zhangwuia and Cretaceous Foxeoidea does not constitute a serious problem for this interpretation, as Zhangwuia and Foxeoidea may belong to two different parallel lineages.
Parallel to Zhangwuia and Foxeoidea, a possible Gnetales-related taxon with typical ephedroid morphology, Pseudoephedra (Liu & Wang Reference Liu and Wang2016a), bears a solid style (a feature of angiosperms) instead of a micropylar tube as expected in Ephedra. According to molecular studies (Skinner et al. Reference Skinner, Hill and Gasser2004), the placenta and ovarian wall correspond to an axillary branch and a leaf in gymnosperms, respectively. Foxeoidea, Zhangwuia, and Pseudoephedra seem to suggest that there may be a novel evolutionary path for angio-ovuly (Wang et al. Reference Wang, Liu, Liu, Zhang, Guo, Hu, Zhang, Wang and Liao2015): angio-ovuly may be reached by different plant groups in their own ways independently, as suggested by others previously (Krassilov Reference Krassilov1977; Wu et al. Reference Wu, Lu, Tang, Chen and Li2002). The possibility of deriving conduplicate carpels from the assumed ‘megasporophylls' which bear ovules along their margins, as assumed by Arber & Parkin (Reference Arber and Parkin1907) and their proponents, is reduced to nil since the superficially leaf-like morphology of megasporophyll in Cycas has been experimentally proven to be an artefact due to mechanical pressure (Wang & Luo Reference Wang and Luo2013). Given all the evidence, angio-ovuly seems to have been reached independently.
4. Conclusion
Zhangwuia is a reproductive organ showing a mosaic combination of bennettitalean and angiospermous features. While its lack of exserted micropylar tube makes it atypical in Bennettitales, its ovule enclosed inside the female unit makes Zhangwuia closer to angiosperms. Despite its enigmatic phylogenetic position, Zhangwuia appears to narrow the gap between Bennettitales and angiosperms in an unexpected way.
5. Acknowledgements
We thank Ms Chunzhao Wang for her help with SEM observation. This research is supported by the National Natural Science Foundation of China (41688103, 91514302, 91114201, 41572046), the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (grant no XDB26000000) awarded to X. W.; and State Forestry Administration of China (no. 2005-122), the Science and Technology Project of Guangdong (no. 2011B060400011), and the Special Funds for Environmental Projects of Shenzhen (no. 2013-02) awarded to Z. J. L. This is a contribution to UNESCO IGCP632.
6. Supplementary material
Supplementary material is available online at https://doi.org/10.1017/S1755691018000257









