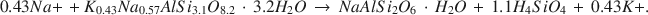After a temperature-dependent period when little dissolution occurs, the dissolution of rhyolitic glass can be described by dC/dt = k(Cs − C), where Cs is the concentration of dissolved silica at saturation, C is the instantaneous silica concentration, and k is a rate constant equal to 1.6 × 10−5, 3.0 × 10−5, and 4.5 × 10−5 sec−1 at 115°, 130°, and 140°C respectively, in 2 M Na-K carbonate solution at 1 kbar pressure. At 130°C, a Cs value of 0.177 M SiO2 is reached in 30 hr, and phillipsite, clinoptilolite, and mordenite begin forming at 34, 64, and 76 hr, respectively, in 2 M CO3, 1:1 Na/K. During glass dissolution and zeolite formation, the concentration of Al as Al(OH)4− is buffered at 3.7 × 10−4 M by an unidentified phase. The ratio of SiO2 to Al(OH)4− at the onset of zeolite formation is 475. In 2 M CO3 solution, phillipsite crystallization begins at 144 hr at 115°C, at 34 hr at 130°C, and at 20 hr at 140°C. Phillipsite crystallization begins at 48 hr in 1.5 M CO3, at 168 hr in 1.0 M CO3, and in excess of 550 hr in 0.2 M CO3 at 140°C. In addition to OH− catalysis, CO32- appears also to catalyze the glass-dissolution and zeolite-formation processes.
Thermodynamically, phillipsite is unstable relative to clinoptilolite and mordenite in silica-rich alkaline hydrothermal solutions. Phillipsite forms first, followed by clinoptilolite, and then mordenite. Phillipsite formation is favored by runs of one-week duration, temperatures less than 150°C, and K-rich fluids. Clinoptilolite formation is favored in runs of more than one week, temperatures less than 150°C and K-rich fluids. Mordenite formation is favored by runs of more than one week, temperatures greater than 140°C, and Na-rich fluids. In 8-day runs at 140°C, clinoptilolite formation was favored by liquid: solid reactant (volume: mass) ratios less than 1.0, mordenite by ratios from 0.85 to 1.5, and phillipsite by ratios greater than 1.5. The mechanism of formation of the different zeolites, particularly phillipsite, may involve silica-cyclic tetramers which are abundant in concentrated solutions under alkaline hydrothermal conditions but which are almost absent in dilute low-temperature solutions. Thus, the results of hydrothermal experiments may not be directly applicable to zeolite formation at low temperatures.