Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Intissar, Mourad
Segni, Rachid
Payen, Christophe
Besse, Jean-Pierre
and
Leroux, Fabrice
2002.
Trivalent Cation Substitution Effect into Layered Double Hydroxides Co2Fey Al1−y(OH)6Cl·nH2O: Study of the Local Order.
Journal of Solid State Chemistry,
Vol. 167,
Issue. 2,
p.
508.
Intissar, Mourad
Jumas, Jean-Claude
Besse, Jean-Pierre
and
Leroux, Fabrice
2003.
Reinvestigation of the Layered Double Hydroxide Containing Tetravalent Cations: Unambiguous Response Provided by XAS and Mössbauer Spectroscopies.
Chemistry of Materials,
Vol. 15,
Issue. 24,
p.
4625.
Ping Xu, Zhi
Braterman, Paul
and
Yarberry, Faith
2004.
Handbook of Layered Materials.
Evans, David G.
and
Slade, Robert C. T.
2005.
Layered Double Hydroxides.
Vol. 119,
Issue. ,
p.
1.
Iglesias, Amadeu H.
Ferreira, Odair P.
Gouveia, Daniel X.
Souza Filho, Antonio G.
de Paiva, José A.C.
Mendes Filho, Josué
and
Alves, Oswaldo L.
2005.
Structural and thermal properties of Co–Cu–Fe hydrotalcite-like compounds.
Journal of Solid State Chemistry,
Vol. 178,
Issue. 1,
p.
142.
Intissar, Mourad
Malherbe, François
Prévot, Vanessa
and
Leroux, Fabrice
2006.
Evidences of segregated SnO2 type nanoparticles coating layered double hydroxide at moderate temperature.
Journal of Colloid and Interface Science,
Vol. 299,
Issue. 2,
p.
747.
Forano, C.
Hibino, T.
Leroux, F.
and
Taviot-Guého, C.
2006.
Handbook of Clay Science.
Vol. 1,
Issue. ,
p.
1021.
Duchet‐Rumeau, Jannick
and
Sautereau, Henry
2010.
Epoxy Polymers.
p.
159.
Bravo-Suárez, Juan J.
Subramaniam, Bala
and
Chaudhari, Raghunath V.
2012.
Ultraviolet–Visible Spectroscopy and Temperature-Programmed Techniques as Tools for Structural Characterization of Cu in CuMgAlOxMixed Metal Oxides.
The Journal of Physical Chemistry C,
Vol. 116,
Issue. 34,
p.
18207.
Jung, Hyangmi
Ohashi, Hidenori
Anilkumar, Gopinathan M.
Zhang, Peilin
and
Yamaguchi, Takeo
2013.
Zn2+ substitution effects in layered double hydroxide (Mg(1−x)Znx)2Al: textural properties, water content and ionic conductivity.
Journal of Materials Chemistry A,
Vol. 1,
Issue. 42,
p.
13348.
Louvain, Nicolas
Peyroux, Jérémy
Dubois, Marc
Simond, Wikenson
and
Leroux, Fabrice
2014.
Efficient Fluorinating Agent through Topochemical Fluorination of Co–Fe Layered Double Hydroxides.
Inorganic Chemistry,
Vol. 53,
Issue. 2,
p.
852.
Zhang, Fengrong
Du, Na
Li, Haiping
Liu, Jianqiang
and
Hou, Wanguo
2014.
Synthesis of Mg–Al–Fe–NO3 layered double hydroxides via a mechano-hydrothermal route.
Solid State Sciences,
Vol. 32,
Issue. ,
p.
41.
Xu, Zhi Ping
and
Braterman, Paul S.
2014.
Dekker Encyclopedia of Nanoscience and Nanotechnology, Third Edition.
p.
2056.
Grégoire, Brian
Greenwell, H. Christopher
and
Fraser, Donald G.
2018.
Peptide Formation on Layered Mineral Surfaces: The Key Role of Brucite-like Minerals on the Enhanced Formation of Alanine Dipeptides.
ACS Earth and Space Chemistry,
Vol. 2,
Issue. 8,
p.
852.
Knorpp, Amy J.
Zawisza, Anna
Huangfu, Shangxiong
Borzì, Aurelio
Clark, Adam H.
Kata, Dariusz
Graule, Thomas
and
Stuer, Michael
2022.
Hydrothermal synthesis of multi-cationic high-entropy layered double hydroxides.
RSC Advances,
Vol. 12,
Issue. 40,
p.
26362.
Eveillard, Fabien
Guérin, Katia
Batisse, Nicolas
Lemoine, Kevin
Rouag, Abdelraouf
Delbègue, Diane
and
Leroux, Fabrice
2023.
Fluorination of (Mg,Cu,Al,Fe)-based LDHs template for conversion materials usable in all-solid state lithium metal batteries.
Applied Clay Science,
Vol. 243,
Issue. ,
p.
107071.
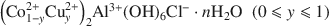 was studied by X-ray diffraction and X-ray absorption spectroscopy. It was found that the lamellar character is maintained over the entire range of the substitution. The local order for the composition {Co2Al} is typical of brucite-like sheets, whereas segregation into small domains may explain the results obtained when the percentage of Cu atoms is increased. The {Cu2Al} end-member material presents a local order around the Cu atoms closely related to the botallackite structure as present in basic layered Cu salts, with the presence of two distinct Cu-Cu distances.
was studied by X-ray diffraction and X-ray absorption spectroscopy. It was found that the lamellar character is maintained over the entire range of the substitution. The local order for the composition {Co2Al} is typical of brucite-like sheets, whereas segregation into small domains may explain the results obtained when the percentage of Cu atoms is increased. The {Cu2Al} end-member material presents a local order around the Cu atoms closely related to the botallackite structure as present in basic layered Cu salts, with the presence of two distinct Cu-Cu distances.

