Case presentation
A 3-month-old male weighing 4.8 kg was born with truncus arteriosus, interrupted aortic arch, and persistent left superior caval vein draining into the right atrium via what was initially thought to be fully roofed coronary sinus. He underwent surgical repair on day 10 of life consisting of end-end arch repair, ventricle septal defect closure, and placement of right ventricle-pulmonary artery homograft. Immediately after repair, stents were placed in the proximal right and left pulmonary arteries due to severe stenosis. Due to the persistent low oxygen saturation (in the mid-80s), a bubble study echocardiogram was performed which revealed that the coronary sinus was partially unroofed. A cardiac CT scan 3 weeks post-repair showed stenosis of the left pulmonary artery proximal to the stent, as well as normal size common right and common left pulmonary veins. An echocardiogram at 3 months of age revealed increased estimated right ventricle systolic pressure, increased flow velocity in the branch pulmonary arteries, flow turbulence in the common left pulmonary vein, no atrial septal defect, dilated coronary sinus ostium, and unroofed distal coronary sinus with the unroofed segment measuring 7 mm in length (Figs 1 and 2). He was taken to the cardiac catheterisation lab for possible intervention on the stented pulmonary arteries. The right femoral vein and artery were accessed using a 5-Fr sheath and a 20-gauge catheter, respectively. Haemodynamic measurements showed elevated distal pulmonary artery pressure with a mean of 26 mmHg and an elevated calculated pulmonary vascular resistance (9.8 Wood units × m2). Balloon angioplasty was then performed in the right and left pulmonary artery stents, along with the placement of an additional stent in the proximal left pulmonary artery to treat the aforementioned stenosis. The left pulmonary artery wedge angiogram showed severe ostial stenosis of the common left pulmonary vein (Fig 3A). Attempts to cross the atrial septum (probing the foramen ovale) were unsuccessful. Knowing the patient had an unroofed coronary sinus, we elected to attempt left atrial access via the coronary sinus. A coaxial system consisting of a 5-Fr JR4 guide catheter (Medtronic), 4-Fr JR 2 catheter (Cook Medical), and 0.035" angled glide wire (Terumo) was inserted through the right femoral vein sheath into the coronary sinus. The catheters were oriented superiorly and rightward. The wire was advanced to cross the coronary sinus into the left atrium. The catheters were then advanced over the wire inside the left atrium. The angled glide wire was exchanged for a 0.014" Choice wire (Boston Scientific). The catheters were turned toward the common left pulmonary vein. The Choice wire was manipulated to cannulate the common left pulmonary vein. The JR2 catheter was then advanced over the wire into the left lower pulmonary vein (Fig 2). Pressure measurements revealed a gradient of 16 mmHg across the stenosis. Angiograms showed severe stenosis of the left pulmonary vein just distal to the ostium and the normal pulmonary vein measuring 4.2 mm (Fig 3B). The Choice wire was exchanged for a stiffer coronary wire [0.014" Asahi Grand Slam wire (Abbott)] to provide more support for pulmonary vein stent placement. Then, the 5-Fr guide catheter was advanced over the JR2 catheter inside the vein and the JR2 catheter was removed. A 4 × 8 mm Synergy everolimus-eluting stent (Boston Scientific) was advanced over the wire inside the guide catheter and centred across the stenotic portion. The stent was deployed by inflating the balloon to 18 atmospheres (the rated burst pressure) to achieve a stent diameter slightly larger than 4 mm to match the normal distal pulmonary vein segment. Angiography revealed a well-positioned stent (Fig 3C). Pressure measurement showed no gradient across the stent. No significant improvement in his oxygen saturation was noticed at the end of the procedure. The patient was discharged home on the same home dose of aspirin and started on Sildenafil. Over the next few weeks following the procedure, his oxygen saturation has increased to 93–95%.
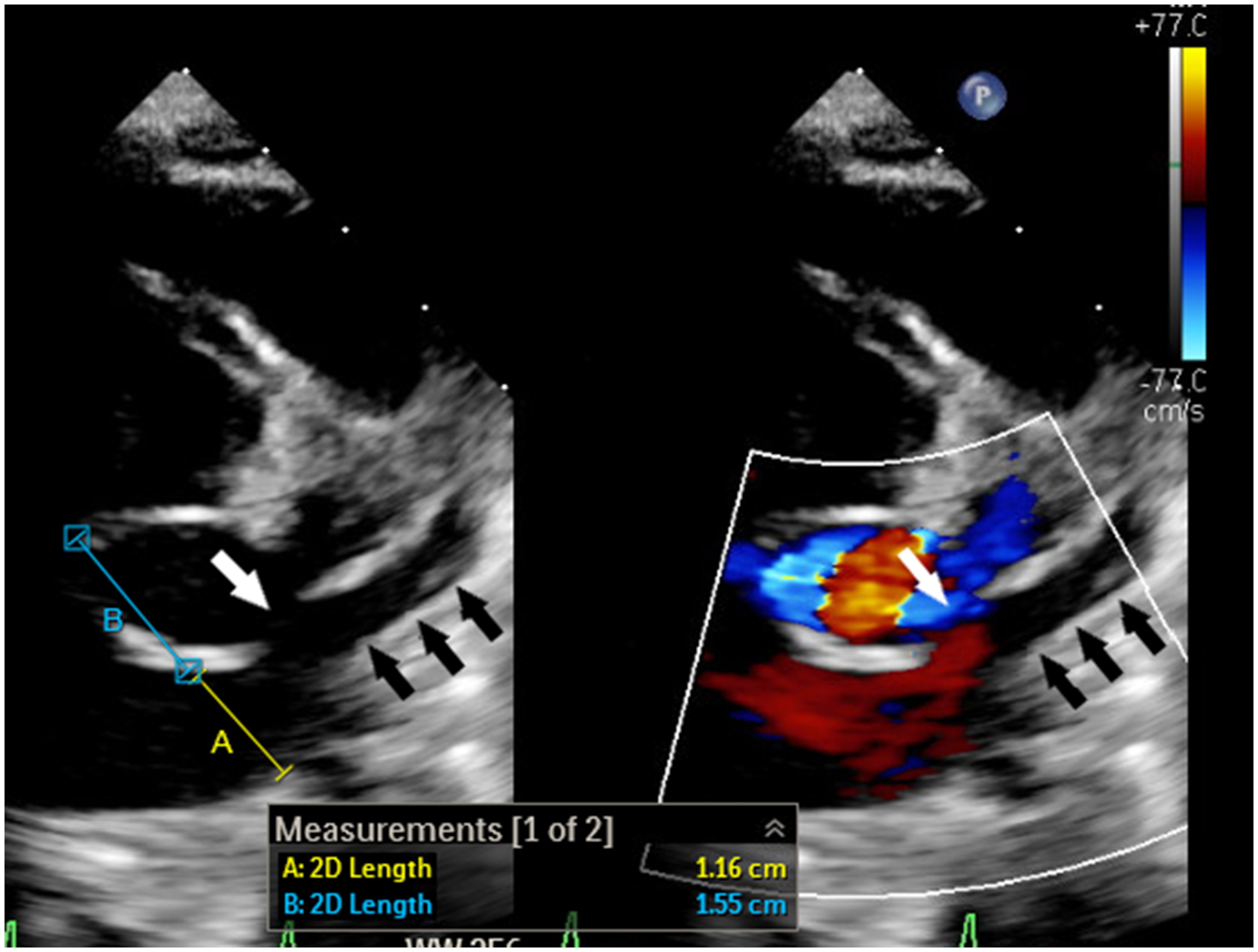
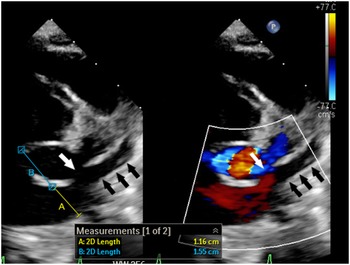
Figure 1. 2-Dimensional echocardiogram with and without colour shows dilated coronary sinus ostium (A) with short septal length (B) and partially unroofed coronary sinus (black arrows). The unroofed segment is depicted with the white arrow.

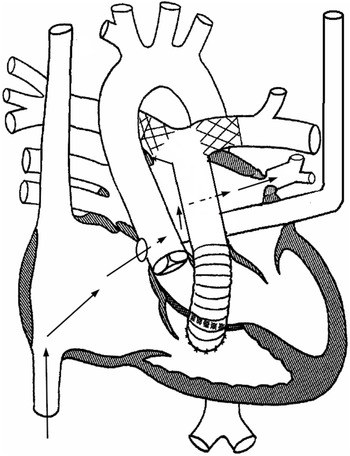
Figure 2. Schematic diagram explaining the anatomy. Black arrows depict the course that was utilised to access the stenotic pulmonary vein. The catheters were advanced from the right atrium coronary sinus ostium unroofed coronary sinus left atrium left upper pulmonary vein.
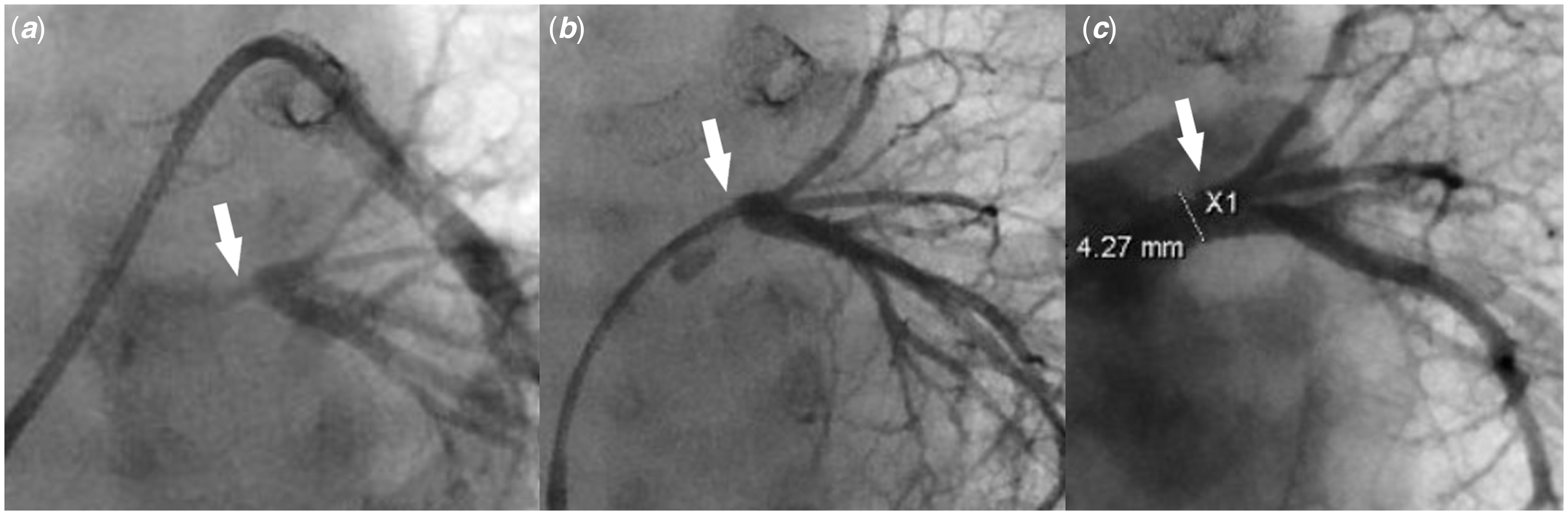
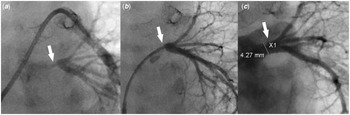
Figure 3. (a) Pulmonary artery wedge angiogram shows severe left common pulmonary vein ostial stenosis (white arrow). ( b) Left common pulmonary vein angiogram shows severe left common pulmonary vein stenosis (white arrow) with well-developed distal pulmonary vein branches. ( c) Left pulmonary vein angiogram post-stent placement shows a well-positioned stent (white arrow) inside the left common pulmonary vein with good expansion, resolution of the stenosis, and no evidence of vascular injury or jailing of the segmental left pulmonary veins.
Discussion
Despite the advancement in surgical and transcatheter treatments, the prognosis of extensive primary pulmonary vein disease continues to be poor with an estimated mortality rate of 40–50% at 1 year. Reference Vanderlaan, Rome, Hirsch, Ivy and Caldarone1–Reference Khan, Qureshi and Justino4 When the atrial septum is intact, transcatheter pulmonary vein intervention requires creating an atrial septal defect which is challenging and carries substantial risks, especially in young infants. Reference Srinivas, Singla, Reddy, Nagesh and Nanjappa5 Moreover, the presence of the dilated coronary sinus further complicates the safety of the procedure as the septal length becomes significantly shorter (Fig 1). Reference Muller, Backhoff and Schneider6 In the described case, the coaxial system of the 5-Fr JR 4 guide, 4-Fr Jr 2 catheter, and guidewires allowed us to cross the unroofed coronary sinus into the left atrium and the stenotic common left pulmonary vein, with relative ease. In addition, the 5-Fr guide catheter (with an outer diameter of 1.67 mm) enabled us to deploy the stent without the need to exchange for a long sheath. Due to the antiproliferative effect, drug-eluting stents slow the progression of pulmonary vein disease when compared with bare-metal stents. Reference Khan, Qureshi and Justino4 Although exclusive balloon angioplasty is an attractive treatment option, the likelihood of complete occlusion is higher when compared with stent placement. Reference Buiatti, von Olshausen and Martens7 As our patient has a common left pulmonary vein, occlusion would result in complete loss of the physiologic function of the entire left lung which could lead to persistent pulmonary hypertension. Additionally, the presence of a stent will ease transcatheter recanalization should future occlusion occurs as the stent is easily visualized by fluoroscopy. For the future management of the patient, we are planning to perform serial dilation, and we anticipate the need to intentionally fracture the stent to accommodate for somatic growth if a larger diameter is needed. Reference Khan, Qureshi and Justino4
Many features make our case unique. The association of pulmonary vein stenosis in patients with truncus arteriosus and unroofed coronary sinus has never been described in the literature. Reference Feins, Ireland and Gauvreau2,Reference Kalfa, Belli and Bacha3 Additionally, the exact mechanism of pulmonary vein stenosis is unknown. However, it falls under the category of primary pulmonary vein stenosis as it affected a vein that has never been operated on. Reference Vanderlaan, Rome, Hirsch, Ivy and Caldarone1 The presentation of pulmonary vein disease was late, as imaging 3 weeks later post-repair showed no pulmonary vein stenosis. Primary pulmonary vein stenosis in patients with associated CHD is usually either identified prior to or immediately after the surgical repair and is usually attributed to the technique of repair. Reference Feins, Ireland and Gauvreau2,Reference Kalfa, Belli and Bacha3 Furthermore, the presence of an unroofed coronary sinus allowed for safe access to the left atrium without the need to create a new atrial septal defect. To our knowledge, this is the first time such an approach has been described.
Conclusion
Trans-coronary sinus access in patients with an unroofed coronary sinus should be considered for intervention on the left atrium or pulmonary vein if no other atrial communication is present. Large-scale studies are needed to guide the management and improve the outcome of pulmonary vein stenosis in children
Acknowledgements
None.
Financial support
This project received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of interest
None
Ethical standards
No specific ethical approval from Institutional Reviews Boards is necessary for this type of publication. The authors assure that all patient data provided in this case report are anonymised.