Critical aortic stenosis in the neonate is a deadly disease if left untreated. Treatment faces several challenges, the most difficult one being the decision between a biventricular or univentricular pathway in cases with a borderline left ventricle. In neonates who meet the prerequisites for a biventricular circulation, the primary goals, apart from saving the child’s life, are to reduce the stenosis and limit the risk of aortic regurgitation while preserving the native aortic valve.
In the early era of paediatric congenital cardiac surgery, the transventricular approach of dilating the aortic valve was used, followed by valvotomy as an open-heart surgery first described in 1969.Reference Marquis and Logan1,Reference Coran and Bernhard2 Balloon aortic valvulotomy in children was described by Labadidi in 1983,Reference Lababidi, Wu and Walls3 and in neonates and children during the first 2 months of life by Rupprath and Neuhaus in 1985.Reference Rupprath and Neuhaus4 Results have improved considerably over time.Reference Hill, Ginde, Rios, Frommelt and Hill5–Reference Donald and Konstantinov8
A major problem when comparing the results in different studies is the inconsistency in definitions of critical valvular stenosis in the newborn.Reference Freedom, Yoo, Mikailian and Williams9 Another problem is the great variation in follow-up time when evaluating mortality and reinterventions. In isolated critical valvular aortic stenosis, the choice of primary intervention is mainly between surgical aortic valvotomy and balloon aortic valvotomy, although aortic valve replacement in the newborn may also be an option in occasional cases, by homograft insertion or the Ross procedure.Reference Brancaccio, Polito and Hoxha10,Reference Woods, Pasquali and Jacobs11
Given the lack of randomised studies and reviews specifically addressing the outcome of these interventions for isolated critical valvular aortic stenosis in the neonate, it is important to present contemporary results from an unselected cohort. The aim of this study is to describe short-term and long-term survival, reinterventions, and aortic valve replacement in a complete national cohort of neonates with intervention before 30 days of age, using surgical aortic valvotomy as the preferred initial treatment.
Patients and methods
We included all patients under 1 month of age who underwent intervention for critical valvular aortic stenosis between 1 January, 1994 and 31 December, 2016, at the two centres for paediatric heart surgery in Sweden. Since 1994, all paediatric heart surgery in Sweden has been centralised to the Queen Silvia Children’s Hospital, Sahlgrenska University Hospital, Gothenburg, and the Pediatric Heart Center at Lund University Hospital, Lund. All paediatric heart surgery and paediatric catheter interventions on aortic valves in Sweden are carried out at these two centres, up to 18 years of age. Data were collected from patient files at the two centres and at the referring hospitals, and patients were identified in the surgical and catheter registries. Survival was cross-checked against the Swedish Population Registry as of December 2017. Reinterventions were cross-checked with the Swedish Registry of Congenital Heart Disease.
Critical aortic stenosis was strictly defined as severe valvular stenosis with duct-dependent systemic circulation and/or depressed left ventricular function with fractional shortening below 28%.Reference McCrindle, Blackstone and Williams7,Reference Freedom, Yoo, Mikailian and Williams9 Neonates with isolated valvular aortic stenosis without duct dependency and with preserved left ventricular function treated before 30 days of age were thus excluded. Patients with associated cardiac malformations requiring concomitant surgery at primary treatment were also excluded, and only patients primarily assigned to biventricular repair were included. The preferred initial treatment in both institutions is surgical valvotomy, which is commissurotomy including thinning of dysplastic leaflets and shaving off noduli when appropriate. Balloon valvotomy as first intervention was performed during a limited period of time (2000–2006) in one of the centres. Also, in the early period (1995–1996), closed transventricular valvotomy was performed in two premature babies with low birth weights.
Transthoracic echocardiography was used for evaluating anatomy, Doppler-derived gradients, aortic valve regurgitation, and left ventricular function. Doppler gradients were derived using the modified Bernoulli equation for the maximal gradient. Mean gradient was calculated from the traced velocity curve. Aortic regurgitation was graded 0–4 based on the colour Doppler jet and retrograde flow in the descending aorta. For assessment of left ventricular function, ventricular end-diastolic diameter and left ventricular fractional shortening were used. The pre-operative echocardiography registrations were re-examined to ascertain that all patients had fulfilled our criteria of critical aortic stenosis and to systematically diagnose and grade left ventricular endocardial fibroelastosis. The presence of endocardial fibroelastosis was graded as 0 (no), 1 (focal), or 2 (extensive). The main indication for reintervention was a Doppler mean gradient or invasive gradient above 50 mmHg or significant aortic regurgitation. Additional criteria were chest pain or fatigue, ischemic electrocardiographic changes, pathological bicycle ergometry test, or left ventricular dysfunction. For evaluation of aortic regurgitation, magnetic resonance tomography was also used.
Statistical analysis
SPSS statistical software was used for data analysis. Categorical variables were reported as absolute numbers and percentages. Continuous variables were expressed as either mean ± standard deviation or median value and range. For all tests, p < 0.05 was considered statistically significant. Survival and freedom from reintervention were calculated using the Kaplan–Meier method.
Results
We identified 117 patients with treatment of isolated valvular aortic stenosis before 1 month of age, between 1 January, 1994 and 31 December 2016. Of those, 41 did not fulfill our criteria for a critical aortic stenosis and were therefore excluded from further analysis, as were 15 patients who had been primarily selected for the univentricular route.
Hence, the study cohort consisted of 61 neonates with isolated critical valvular aortic stenosis, 32 and 29 at the two centres, respectively. There were 50 boys and 11 girls. All medical files were retrieved with no patient lost to follow-up. Median follow-up time was 11.8 years (1.1–23.8). Patient characteristics and echocardiographic variables are shown in Tables 1 and 2. There were no statistically significant differences at group level regarding patient characteristics between the two centres, and the proportion of patients with fractional shortening under 28% was identical (79%). Duct dependency was present in 36 cases (59%). Extensive endocardial fibroelastosis was found in 11 patients, who all had severe left ventricular dysfunction at presentation. Of the remaining patients, 22 had focal and 24 no endocardial fibroelastosis. In four cases, the echocardiogram was not retrieved. Symptoms at presentation were tachypnea and/or a heart murmur at routine examination at the maternity ward. No patient presented with circulatory collapse. Prenatal diagnosis was seen in two cases.
Table 1. Patient characteristics
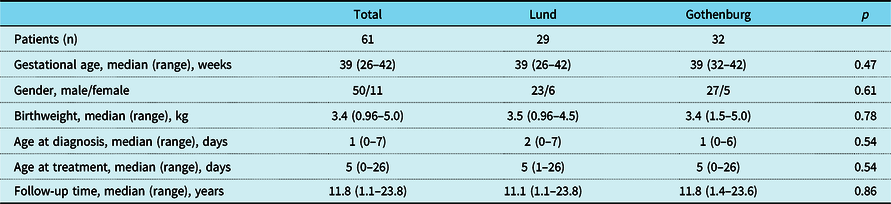
The Kruskal–Wallis test was used to measure differences between patient groups.
Table 2. Echocardiographic and catheter-derived data before and after primary intervention
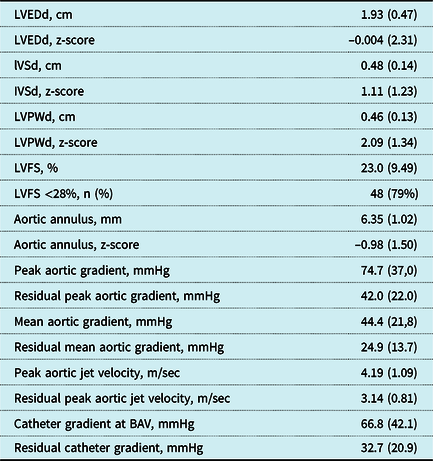
Values represent mean (standard deviation), except where otherwise stated. LVEDd = left ventricular end-diastolic dimension, IVSd = interventricular septum end diastole, LVPWd = left ventricular posterior wall end diastole, LVFS = left ventricular fractional shortening, BAV = balloon aortic valvotomy. All patient records were reviewed for LVFS and duct dependency, but in 11 patients the complete echocardiographic data could not be retrieved.
Surgical valvotomy was the initial procedure in 52 patients. At one centre, all patients had a surgical valvotomy (n = 29) and at the other centre, initial treatment was surgical valvotomy (n = 23), balloon valvotomy (n = 6), closed transventricular valvotomy (n = 2), or Ross procedure (n = 1). Figure 1 shows a flowchart with an overview of all interventions. Median age at initial treatment was 5 days (range 0–26).

Figure 1. The flowchart illustrates type of primary intervention, all subsequent reinterventions, and mortality. After the primary procedure, 33 patients had a second, 13 a third, seven a fourth, and 1 a fifth and sixth intervention. Red rings illustrate failure of biventricular strategy.
There was no 30-day mortality. Four late deaths occurred. They were all boys with duct-dependent systemic circulation, extensive endocardial fibroelastosis, poor left ventricle function, and a small aortic valve anulus (mean z-score −1.6) at presentation. One boy with non-apex-forming left ventricle and pulmonary hypertension had a surgical valvotomy but died at 2 months of age of heart failure and an infection. Another boy died at 10 months of age, after repeated balloon and surgical valvotomy, of a concomitant pulmonary infection. A third boy, born with a severely dilated, poorly functioning left ventricle, and severe pulmonary hypertension, had primary surgery with a commissurotomy followed by a Konno procedure with homograft aortic root replacement. Rapid degeneration of the homografts led to repeated homograft insertions. Death occurred at 2 years of age while on ventricular assist, due to severe left ventricular failure. The fourth patient with a biological valve prosthesis died at 21 years of age due to endocarditis and septic cerebral embolisation. Conversion to a single-ventricle palliation was performed in two patients at 10 and 66 days of age, respectively, and two further patients had a heart transplantation due to restrictive left ventricular physiology at age 1.6 and 15 years, respectively. Transplant-free survival is shown in Figure 2.
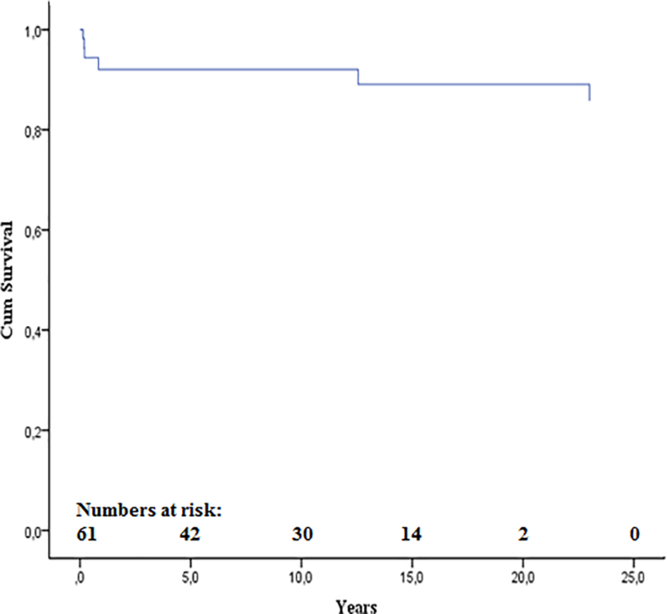
Figure 2. Kaplan–Meier curve of transplant-free survival
A total of 54 aortic valve reinterventions were performed in 29 patients, excluding patients converted to single-ventricle palliation and heart transplantation. Freedom from reintervention in the whole group was 66%, 61%, 54%, 49%, and 46% and after surgical valvotomy 69%, 63%, 56%, 52%, 48% at the age of 1, 5, 10, 15, and 20 years, respectively (Figure 3). Median time to reintervention after surgical valvotomy was 4.9 months (10 days–17.3 years) and after balloon valvotomy was 2.0 months (17 days–3.4 months). All but one of the balloon valvotomy reinterventions were performed in one of the centres. No statistically significant difference was seen in the time from first intervention to reintervention between the two centres (p = 0.061). The most common indication for a second intervention was residual stenosis (Figure 4). There was no association between the presence of endocardial fibroelastosis, focal or extensive, and need for reintervention.

Figure 3. Freedom from reintervention after surgery versus balloon dilatation as primary intervention. SAV = surgical aortic valvotomy, BAV = balloon aortic valvotomy.

Figure 4. Indications for reintervention. Other* includes the total burden of cardiothoracic surgical procedures, that is, Atrial septal defect closure (n = 1), Persistent ductus arteriosus closure (n = 1), pacemaker due to post-operative atrioventricular block (n = 1), replacement of homograft in pulmonary position (n = 1), conversion to single-ventricle palliation with Norwood (n = 1) or Damus–Kaye anastomosis (n = 1) followed by bidirectional Glenn (n = 2) and Total cavopulmonary connection (n = 2), mechanical prosthesis in mitral valve (n = 2), initiating or removal of Left ventricular assist device system (n = 3), and heart transplant (n = 2).
Aortic valve replacement was required in 23 patients (38%) (Figure 1), and seven patients had a repeated valve replacement. Kaplan–Meier curves showed no significant difference in time to aortic valve replacement between the two centres (p = 0.656; Figure 5). Indications for valve replacement are shown in Figure 6. The 11 Ross procedures included 1 primary neonatal intervention, while the remaining 10 were reinterventions, 2 in infants (under 1 year of age) and 8 in children between 3 and 14 years of age. Only one Ross patient had a pulmonary homograft replacement. Homograft insertion in the aortic root position was used as valve replacement in five infants, due to residual stenosis in four cases and combined stenosis and regurgitation in the fifth one. Degeneration of the homograft led to repeated valve replacements in four of these five patients. An 8-year-old boy with aortic regurgitation also had a homograft, when the intended Ross procedure could not be performed. During the study period, 10 of the 11 patients who reached the 18 years of age had an aortic valve replacement during childhood. Figure 7a and 7b illustrates first and last intervention during this follow-up.
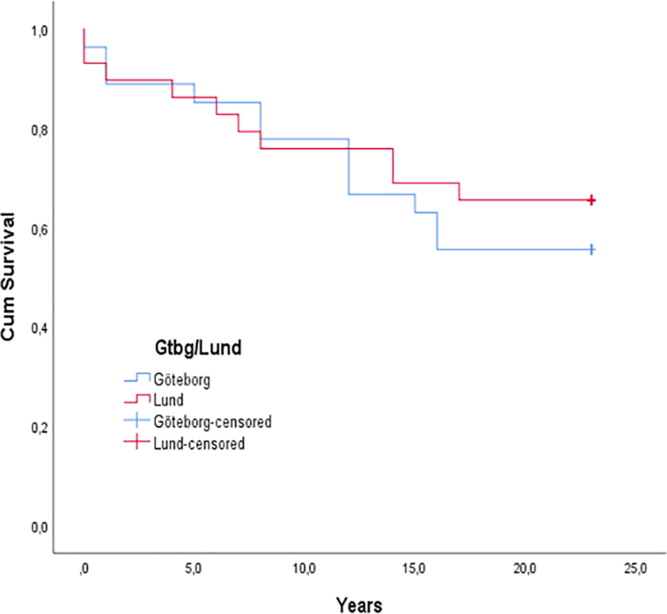
Figure 5. Kaplan–Meier curve of freedom from aortic valve replacement. A Mantel–Cox log-rank test showed no statistically significant difference in time to aortic valve replacement between the two centres (Gothenburg and Lund), p = 0.656.
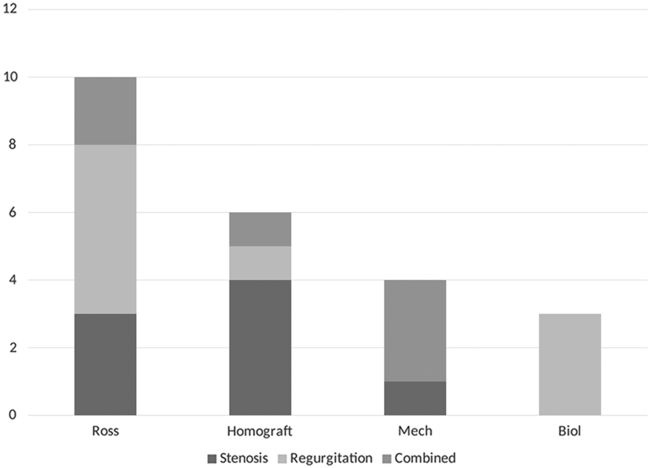
Figure 6. Indications for aortic valve replacement.

Figure 7. Pie chart showing type of initial intervention in all 61 patients (a) and status at follow-up of the 57 patients alive (b). CTV = closed transventricular valvactomy, SAV = surgical aortic valvotomy, BAV = balloon aortic valvotomy, HTx = heart transplantation, Mech prosth = mechanical prosthesis, Biol prosth = biological prosthesis.
Discussion
The purpose of the present study was to describe the outcome of a complete national cohort of neonates treated for isolated critical aortic stenosis. In the present study, we used a strict definition of critical aortic stenosis as severe valvular stenosis with either a duct-dependent systemic circulation or a depressed left ventricular function of such a degree that one could anticipate the patient would die during the neonatal period if left without treatment. Despite the severity of disease, there was no 30-day mortality. There were only four late deaths, and no mortality in association with a reintervention procedure giving a long-term transplant-free survival of 93%. We excluded neonates with a preserved left ventricular function but still treated surgically or by catheter during the first month of life, as they constituted a more benign subgroup.
The main treatment strategy in the present study was surgical valvotomy, but balloon valvotomy was the first intervention in six cases during a limited time period in one of the centres. Observing a high rate of early reintervention after balloon valvotomy (Figure 3), the team decided to abandon this procedure in favour of surgical valvotomy as the primary treatment, an experience in line with Siddiqui et al.Reference Siddiqui, Brizard and Galati12
Early mortality after surgical valvotomy in critical aortic stenosis in the newborn has been reported to be 4.5 to 19%.Reference Siddiqui, Brizard and Galati12–Reference Agnoletti, Raisky and Boudjemline15 A French single-centre study by Galoin-Bertail et al. on a series of 56 neonates treated with surgical valvotomy reported an early mortality of 9% and a total mortality of 13%.Reference Galoin-Bertail, Capderou, Belli and Houyel13. Siddiqui et al., in a study from Melbourne, reported an early mortality of 4.5% in neonates after surgical or balloon valvotomy.Reference Siddiqui, Brizard and Galati12 Our result of a long-term transplant-free survival of 90.2% compares well with a study by Hraska et al. showing 91.2% survival after surgical valvotomy in 34 neonates with critical aortic stenosis, with a mean follow-up of 11 years.Reference Hraska, Sinzobahamvya and Haun14 Early mortality after balloon valvotomy was reported in a large contemporary study, based on data from the American College of Cardiology’s IMPACT registry, with a 30-day mortality of 6.3% and in-hospital mortality of 10% in 110 neonates with strictly defined critical aortic stenosis.Reference Boe, Zampi and Kennedy16 In another large multicentre study, by Ewert et al, 30-day mortality was 8.7% for 334 neonates who had balloon valvotomy.Reference Ewert, Bertram and Breuer6 However, in these two latter studies, no information on late deaths was given. It is also unclear whether all neonates in the above-mentioned studies had critical aortic stenosis; furthermore, patients with associated cardiac defects were commonly included.
As in many other reports, the rate of early reinterventions is substantial. It reflects the complexity of the disease: the more severe the condition at birth, the greater the probability that reinterventions will be necessary (Figure 1). In our study, the 10-year freedom from reintervention after surgical valvotomy was 56% and the first reintervention often occurred early in life (Figure 3). Considering the strict definition of critical aortic stenosis in our cohort, we find this result satisfactory in comparison with other studies. The lack of uniform criteria for reintervention decisions is a complicating factor when comparing long-term outcomes, especially if a mixed valve disease is present with both valvar stenosis and regurgitation. In the current literature, a longer freedom from reintervention is generally reported after surgical valvotomy than after balloon valvotomy, with freedom from reintervention at 10 years after surgical valvotomy of between 35 and 68%.Reference Hill, Ginde, Rios, Frommelt and Hill5,Reference Donald and Konstantinov8,Reference Hraska, Sinzobahamvya and Haun14,Reference Agnoletti, Raisky and Boudjemline15,Reference Alexiou, Chen and Langley17 This was confirmed in a recent publication by Vergnat et al.Reference Vergnat, Asfour and Arenz18 In our study, statistical comparison between surgical and balloon valvotomy is not fruitful, as only six patients had a primary balloon valvotomy.
A major issue is the necessity of aortic valve replacement, with different treatment options available in different age groups. Use of the Ross procedure in the neonatal period is highly disputed because of the high mortality reported in this age group.Reference Brancaccio, Polito and Hoxha10,Reference Woods, Pasquali and Jacobs11,Reference Kallio, Pihkala, Sairanen and Mattila19,Reference Tan Tanny, Yong and d’Udekem20 Valve replacement with aortic root homograft insertion or biological prosthesis usually achieves initially good haemodynamic results without the need for anticoagulation therapy, but with the risk of rapid degeneration requiring repeated replacements.Reference Karamlou, Jang and Williams21,Reference Etnel, Elmont and Ertekin22 A single-centre follow-up and risk analysis by Karamlou of 160 children after aortic valve replacement showed a mortality of 19% and repeated valve replacement in 34% at 10 years after the initial replacement. In our study, the 10-year freedom from aortic valve replacement was 75% but only one of 11 patients entered adulthood with a preserved native valve. We find that the goal to postpone valve replacement until the child is fully grown can be achieved in many patients making a mechanical prosthesis a possible option. No early mortality was associated with aortic valve replacement but two of the late deaths were associated with a previous valve replacement. As in the Karamlou study, 30% (n = 7) had a repeated valve replacement, following a previous homograft insertion or biological prosthesis in aortic valve position.
An interesting finding in the present study is that, although the patients to some extent were offered different methods to relieve residual stenosis of the valve, namely, repeated surgical valvotomy at one centre and balloon valvotomy at the other, the time from first intervention until valve replacement became necessary was the same in the two centres (Figure 4). There is little information in the literature regarding balloon valvotomy as reintervention for relief of residual stenosis after surgical valvotomy in neonates and children with congenital aortic stenosis. To our knowledge, the only report is the publication by Petit at al. that describes outcome of balloon dilatation after surgical valvotomy in 23 children.Reference Petit, Maskatia, Justino, Mattamal, Crystal and Ing23 They suggest that reintervention with balloon valvotomy is both effective in relieving stenosis and delays further surgical interventions. Our experience is that surgical valvotomy as primary treatment has definite advantages but, in this setting, balloon valvotomy may be a good option, as a less invasive procedure, for reintervention to postpone aortic valve replacement. Another important issue that needs to be addressed in the future is if it is possible to identify factors that determine what patients would benefit from earlier Ross procedure rather than repeated valve reinterventions with balloon or surgical valvotomy.
Strengths and limitations
The present study is population-based, as paediatric cardiac surgery in Sweden has been concentrated to two centres since 1994, with no private alternative for paediatric heart surgery available. The strength of this material is the unselected nature of the patient cohort. It includes all neonates requiring treatment for critical aortic stenosis in Sweden during the study period, avoiding potential referral bias or patient selection. Inclusion was made on a strict definition of the diagnosis based on a review of the pre-operative echocardiography registration. We present both short-term and long-term data on survival and reinterventions, with no patient lost to follow-up. One limitation is the retrospective design, and another is that, although a complete national cohort is presented, the number of patients is small.
Conclusion
This study shows that critical aortic stenosis in the neonate may be treated with excellent operative and long-term survival. Despite the severity of the diagnosis, there was no 30-day mortality and long-term survival was 93% with surgical valvotomy as preferred first intervention. Many patients needed a reintervention during childhood, most commonly related to residual stenosis. The effect of balloon valvotomy to relieve residual stenosis was as effective as surgical valvotomy to postpone aortic valve replacement. More than half of the patients were still living with their native aortic valve at end of follow-up.
Acknowledgements
None.
Financial support
This study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (SU 2018-04267), and from the Research and Development Department, Västernorrland County Council.
Conflicts of interest
The authors declare no conflict of interests.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Regional Ethics Review Board of Western Sweden (approval no. 518-16).











