Acute kidney injury after cardiac surgery in adults and children using cardiopulmonary bypass is common. It occurs in up to 40% of these patients, depending on the definition used. Reference Akcan-Arikan, Zappitelli, Loftis, Washburn, Jefferson and Goldstein1,Reference Hoste, Clermont and Kersten2 CPB-related AKI has been consistently shown to increase both morbidity and mortality, Reference Kist-van Holthe tot Echten, Goedvolk and Doornaar3,Reference Kuitunen, Vento, Suojaranta-Ylinen and Pettilä4 even beyond the perioperative period. Reference Brown, Kramer, Coca and Parikh5 Recent studies in both adult and paediatric cardiac surgery patients have identified mild post-operative serum creatinine rise as a risk factor for mortality. Reference Lassnigg, Schmid and Hiesmayr6,Reference Zappitelli, Bernier and Saczkowski7
The Acute Dialysis Quality Initiative issued a consensus-based, multidimensional, graded classification system known as RIFLE (acronym for Risk, Injury, Failure, Loss, and End-Stage Kidney Disease) to classify AKI in 2004. Reference Akcan-Arikan, Zappitelli, Loftis, Washburn, Jefferson and Goldstein1 These criteria have been validated as a predictive value for mortality in studies encompassing different patient populations, including cardiac surgery patients. Reference Mehta, Kellum and Shah8 A paediatric modified version of this classification system, pRIFLE, was developed and validated in 2007. Reference Akcan-Arikan, Zappitelli, Loftis, Washburn, Jefferson and Goldstein1 The pRIFLE criteria system is the most sensitive test for detecting early AKI, especially in the infant age group and in the early identification of AKI in paediatric ICU patients. Reference Lex, Tóth and Cserép9,Reference Khwaja10
Nearly all AKI post-CPB studies in children comprise a heterogeneous population in terms of age, size, and case complexity. Studies of AKI in individual cardiac lesions and after specific cardiac surgery procedures are scarce. To our knowledge, only a few studies have described the incidence and risk factors for severe AKI (according to Acute Kidney Injury Network criteria) in children following the arterial switch operation for transposition of the great arteries. Reference Basu, Andrews, Krawczeski, Manning, Wheeler and Goldstein11–Reference Özker, Saritaş and Vuran13 However, the definition and therefore the incidence of AKI varied significantly between these studies (20% and 51%), as did the complexity of the included TGA patients.
Therefore, the aim of this study was to determine the incidence of AKI according to pRIFLE criteria in children following the ASO for simple and complex TGA patients. Furthermore, we aimed to identify potential risk factors for the development of AKI and to compare characteristics and post-operative outcomes between patients with and without AKI.
Material and methods
We performed a retrospective cohort study of Dutch patients with TGA, who underwent the ASO between January, 2000 and January, 2020. TGA patients with and without ventricular septal defect were included, including those with Taussig–Bing anomaly. Infants with congenital kidney disease were to be excluded.
We retrospectively analysed surgical reports, preoperative neonatal data, and collected post-operative data from the electronic patient data management systems.
The study protocol complied with ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution’s human research committee. As the research was limited to anonymised secondary use of information previously collected during medical care, formal ethical approval was waived.
Preoperative data
Patient data were collected from the day of admission prior to the ASO until PICU discharge. Pre-ASO data included gestational age, age at the time of surgery, pre- or dysmaturity, gender, birthweight, weight at the time of surgery, and baseline creatinine 1 day before the ASO.
Specific preoperative data included associated cardiac anomalies (e.g., VSD, Taussig–Bing anomaly, Reference Konstantinov14 atrial septal defect, patent ductus arteriosus, coronary artery anatomy, aortic coarctation, and aortic arch interruption), dependence of mechanical ventilation, prostaglandin infusion, and extracorporeal membrane oxygenation. Furthermore, surgery prior to the ASO was also identified and registered (e.g., Blalock–Taussig shunt, pulmonary artery banding, surgical atrial septostomy, and repair of aortic coarctation).
Intra- and post-operative data
Duration of CPB and aortic cross-clamping were collected. Post-operatively, we collected time to delayed sternal closure, duration of mechanical ventilation, vasoactive inotrope scores on days 1 and 2 post-ASO, PICU length of stay, mortality, and the occurrence of complications (cardiac arrest, use of temporary pacing, presence of arrhythmia, re-sternotomy, post-operative sepsis). Sepsis was defined clinically as clinical signs and symptoms (fever, hypothermia, circulatory instability, increased inflammatory markers) of an infection for which antibiotics were administered and continued 5–7 days with or without positive blood culture, without an alternative explanation for the symptoms. In order to quantify inotropic support, we calculated the vasoactive inotropic score. Reference Kumar, Sharma and Sethi15 Post-operative data specific to renal function included daily creatinine levels, change in creatinine from baseline, and the need for renal replacement therapy.
Patients were classified as having AKI based on the pRIFLE criteria using the highest post-operative creatinine at the PICU. Reference Akcan-Arikan, Zappitelli, Loftis, Washburn, Jefferson and Goldstein1 Baseline creatinine was measured 1 day before surgery. Estimated creatinine clearance was calculated by Schwartz equation; eCCL = Length (cm) × K (contante)/serum creatinine. Therefore, an increase in creatinine is an equal decrease in the eCCL.
Four categories based on the rise of creatinine according to pRIFLE criteria were used as follows:
-
“normal”, defined as baseline serum creatinine <150% to baseline;
-
“risk”, defined as 1.5× baseline serum creatinine (150–200%);
-
“injury”, defined as 2× baseline serum creatinine (200–300%); and
-
“failure”, defined as 3× baseline creatinine or estimated creatinine clearance <35 ml/min/1.73 m2
Severe AKI was defined as pRIFLE criteria categories “injury” or “failure” (i.e., >200% creatinine rise).
Analysis of data
Statistical analysis for this quantitative data was conducted in consultation with our hospital PICU statistician using the SPSS for Windows (version 26.0.2). Continuous variables were described as medians with ranges. Categorical variables were presented as frequencies and percentages. Baseline comparisons between patients with and without severe AKI were performed using the chi-square or Mann–Whitney U-test depending on distribution.
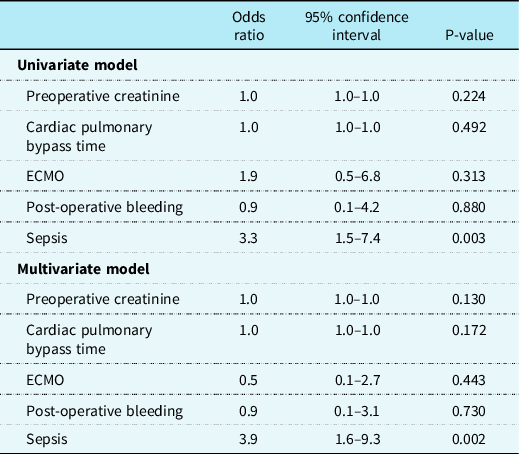
Univariate and multivariate binary logistic regression was performed to identify independent risk factors for developing severe AKI. The following determinants were considered as possible risk factors: preoperative creatinine, CPB duration, use of ECMO, post-operative bleeding, and sepsis. A p-value of less than 0.05 (two-sided) was considered statistically significant.
Results
Incidence of AKI (Fig 1)
Two-hundred and forty-three patients underwent the ASO for TGA. One patient was excluded as mortality occurred directly after the ASO before AKI could develop. Five children (4%) recovered from AKI before the ASO. One-hundred and twenty-three (51%) of the patients developed AKI after the ASO. Sixty-five patients (27%) were classified as Risk, 44 patients (18%) as Injury, and 13 patients (5%) as Failure. From the children in the Failure group, five had an estimated creatinine clearance <35 ml/min/1.73 m2 before the operation and all five children underwent balloon atrial septostomy before the ASO.
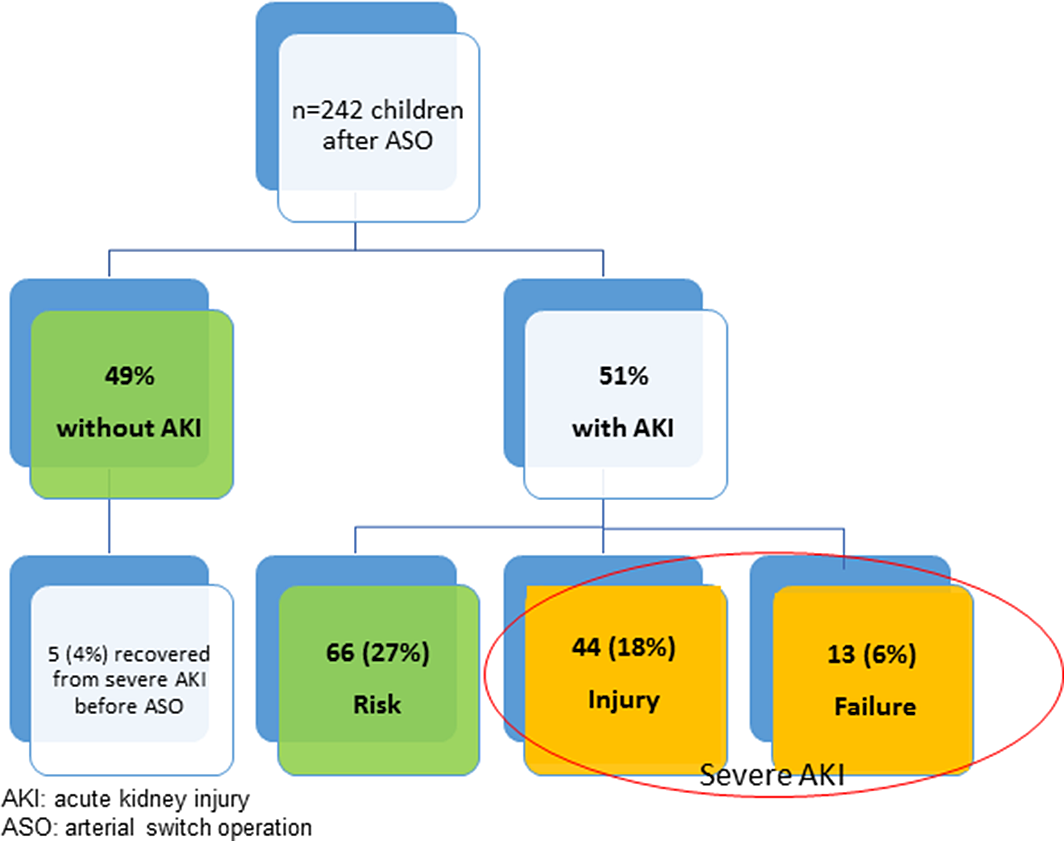
Figure 1. Incidence of acute kidney injury after arterial switch operation.
Fifty-seven patients (24%) were classified as severe AKI. Four children were treated with RRT (continuous veno-venous hemofiltration in one, peritoneal dialysis in four). Eighteen patients (14%) still had AKI at the time of discharge from the PICU. There was no difference in the incidence of severe AKI between the first and last decade of the study (23 versus 24%, p = 0.808).
Preoperative characteristics (Table 1)
Table 1. Preoperative demographic and basic characteristics of children with and without severe AKI after arterial switch operation
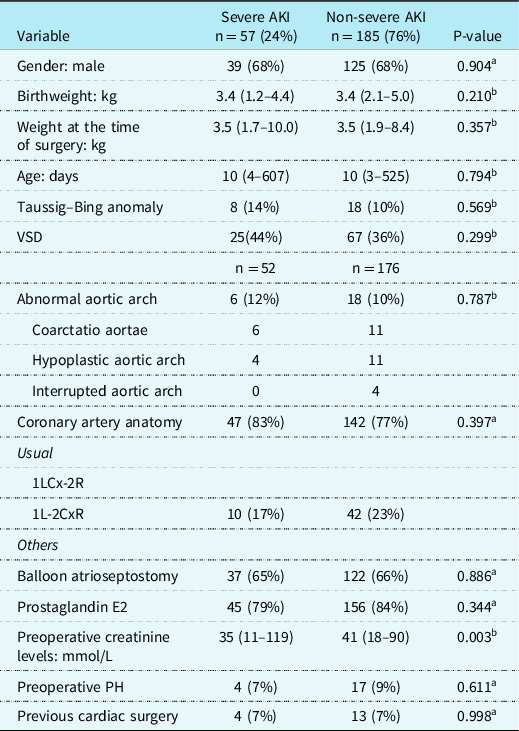
AKI = Acute Kidney Injury; kg = kilograms; PH = Pulmonary Hypertension; VSD = ventricular septal defect.
Data are in median (range) or n (%).
a Pearson’s chi-square test;
b Mann–Whitney U-test.
Preoperative baseline characteristics for all patients with or without severe AKI after the ASO are listed in Table 1. Overall, there were no significant differences in preoperative characteristics (e.g., age, sex distribution, or weight) between the two groups, except for preoperative creatinine levels which were significantly lower in children with AKI, respectively, 35 mmol/L (11–119) versus 41 mmol/L, (18–90), p = 0.003. With univariate analysis, we could not identify preoperative variables as risk factors for the development of AKI after the ASO.
Intraoperative data (Table 2)
Table 2. Intraoperative characteristics of patients with and without severe AKI after arterial switch operation
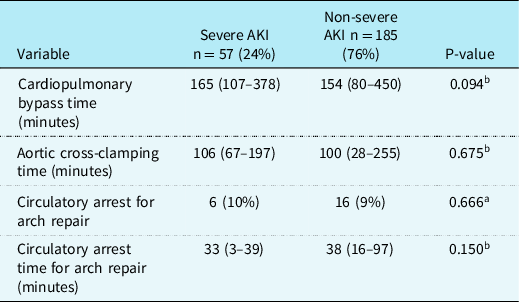
AKI = Acute Kidney Injury.
Data are in median (range) or n (%).
a Pearson’s chi-square test;
b Mann–Whitney U-test.
Intraoperative variables for all patients with and without severe AKI after the ASO are listed in Table 2. There were no significant differences in CPB duration, aortic cross-clamp time, or circulatory arrest for patients with aortic arch repair between patients with and without severe AKI.
Post-operative data (Table 3)
Table 3. Post-operative factors and complications for children with and without severe AKI after arterial switch operation
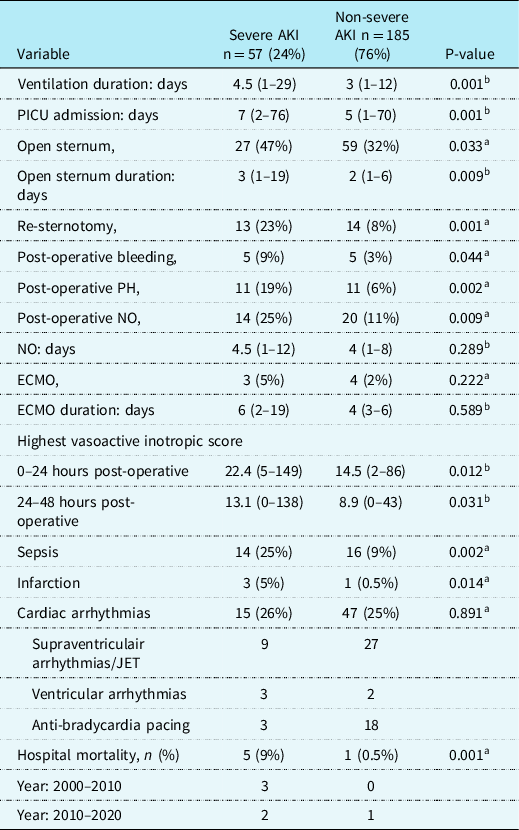
AKI = Acute Kidney Injury; ECMO = extracorporeal membrane oxygenation; JET = Junctional ectopic tachycardia; NO = Nitric Oxide; PICU = paediatric intensive care unit; PH = pulmonary hypertension; POD1 = post-operative day 1; POD2 = post-operative day 2; SVT = supraventricular tachycardia.
Data are in median (range) or n (%).
a Pearson’s Chi-Square test;
b Mann–Whitney U-test.
Post-operative data and complications for all patients with and without severe AKI are listed in Table 3. Amongst the 242 patients, there were 6 (2.5%) in-hospital deaths. Three patients (5%) were supported on ECMO in the AKI group and 4 (2%) in the non-AKI group (p = 0.268). Children with severe AKI had a longer duration of mechanical ventilation, 4.5 (1–29) versus 3 (1–12) days (p = 0.001). Also, the severe AKI group had a higher rate of patients with myocardial ischaemia/infarction, 3 (5%) versus 1 (0.5%) (p = 0.002), pulmonary hypertension, 11 (19%) versus 11 (6%), p = 0.002, post-operative bleeding, 5 (9%) versus 5 (3%), p = 0.044, re-sternotomy 13 (23%) versus 14 (8%), p = 0.001, longer time to sternal closure, 3 (1–19) versus 2 (1–6 days), p = 0.009, higher VIS at 24 and 48 hours post-operative, respectively, 22 (5–149) versus 15 (2–86), p = 0.012 and 13 (0–138) versus 9 (0–43), p = 0.031 and a higher rate of sepsis, 14 (24%) versus 16 (9%), p = 0.002. In children with severe AKI, the in-hospital mortality rate was higher compared to children without severe AKI, 5 (9%) versus 1 (0.5%), p = 0.001. There was no difference in mortality between the first decade and the last decade of the study.
Univariate and multivariate binary logistic regression was performed aimed to identify independent risk factors for developing severe AKI (Table 4). Preoperative creatinine, CPB duration, post-operative bleeding, and post-operative ECMO were not associated with developing severe AKI. Only sepsis was identified as a significant independent risk factor (odds ratio [95% CI] 3.9 [1.6–9.3]).
Table 4. Results of univariate logistic regression analysis for severe AKI after the arterial switch operation
ECMO = extracorporeal membrane oxygenation.
Discussion
It is important to identify AKI after cardiac surgery at an early stage due to high morbidity and mortality. Reference Zappitelli, Bernier and Saczkowski7,Reference Basu, Andrews, Krawczeski, Manning, Wheeler and Goldstein11,Reference Tóth, Breuer and Cserép16 Studies of AKI in paediatric cardiac surgery have been limited to several different definitions of AKI. Zappitelli Reference Zappitelli, Bernier and Saczkowski7 found that even a small increase in the serum creatinine level in the immediate post-operative period predicts the development of AKI according to the pRIFLE criteria and that moderate-to-severe AKI is associated with increased morbidity and mortality. However, nearly all AKI post-CPB studies in children comprise a heterogeneous population for age, weight, and case complexity. Reference Zappitelli, Bernier and Saczkowski7,Reference Li, Krawczeski and Zappitelli17 The use of different definitions of AKI makes it difficult to compare results. Studies of AKI in individual cardiac lesions and after specific operative procedures are scarce. We aimed to study the incidence and risk factors for developing AKI in a specific group of children after the ASO for TGA. Our single-centre study shows a high rate of (severe) AKI after the ASO and an association with increased morbidity and mortality. The incidence of AKI is equivalent to similar studies of AKI in children after the ASO. Reference Basu, Andrews, Krawczeski, Manning, Wheeler and Goldstein11 , Reference Harmer, Southgate, Smith, Bharucha, Viola and Griksaitis12
The development of AKI in our study population may well be multifactorial, but can probably be explained by a state of renal under perfusion before during or after the surgery. More specifically, patient characteristics, complications prior to the ASO, and factors prolonging surgical duration may be of importance.
In addition, post-operative haemodynamic instability and complications (e.g., sepsis, ECMO, etc.) are also of influence. Reference Li, Krawczeski and Zappitelli17 We have retrospectively evaluated these previously described risk factors in our single-centre cohort of patients after the ASO over the past 20 years, and compared the prevalence of these factors between the groups with and without severe AKI. In our study, only clinically suspected infections, for which antibiotic treatment was given and continued on clinical grounds, were associated with the development of AKI and vice versa. Due to the design of our study, we could not determine any causative effect.
Cardiopulmonary bypass, presumably, in combination with ischaemia–reperfusion injury, hypothermia, and surgical trauma, elicits a complex, systemic inflammatory response. Reference Kozik and Tweddell18 It is characterised by activation of the complement cascade, release of endotoxin, activation of leukocytes and the vascular endothelium, and release of pro-inflammatory cytokines. This complex inflammatory state causes a transient immunosuppressed state, which may increase the risk of hospital-acquired infection in these children. Reference SooHoo, Griffin and Jovanovich19 An association of AKI with subsequent infections and/or sepsis has been demonstrated by Soohoo et al, in their study of children with and without AKI after cardiac surgery. Reference SooHoo, Griffin and Jovanovich19 They investigated the association between AKI and infection in children younger than 30 days who underwent the Norwood operation. AKI occurred in 40% of patients. Infection occurred at a median of 6 (IQR: 3–13) days after AKI diagnosis. After adjusting for confounders, there were 3.63 greater odds of developing an infection in those patients with AKI (95% CI: 1.36–9.75).
Increased post-operative creatinine levels allow us to identify the most ill children at an early stage at which appropriate interventions and therapies should be targeted. Zappitelli et al showed that AKI mostly occurs in the first 4 post-operative days. Reference Zappitelli, Bernier and Saczkowski7
After CPB, urine output may remain marginal during the first 48–72 hours post-operatively. Therefore, to avoid volume overload and oedema, early use of diuretics is recommended once adequate renal preload and circulatory volume is ensured. In many centres, it is common practice to insert a peritoneal (Tenckhoff) catheter in the perioperative phase to help alleviate a deficiency of urine output. Li et al showed that early initiation of peritoneal dialysis in children after cardiac surgery is safe and allows for superior fluid management with improved clinical outcomes compared to diuretic administration. Reference Li, Krawczeski and Zappitelli17 Use of prophylactic PD should be strongly considered amongst infants at high risk for post-operative acute kidney injury and fluid overload. Reference Özker, Saritaş and Vuran13 If necessary, consider consulting a paediatric nephrologist early in the progress. Peritoneal catheters should be removed as soon as possible to prevent infection when patients establish adequate urine productivity. Additionally, avoiding nephrotoxic drugs or dosing based on estimated GFR and/or drug levels, could help reduce or avoid renal injury. For specific antibiotics (i.e., vancomycin, gentamycin), careful therapeutic drug monitoring should be performed.
Further research should emphasise on long-term follow-up. Previously, it was assumed that in patients with a single episode of AKI kidney function would fully restore without long-term consequences. However, during the past decade, epidemiologic data from critically ill children and adults have suggested that AKI survivors are at considerable risk of developing chronic kidney disease. Reference Askenazi, Feig, Graham, Hui-Stickle and Goldstein20,Reference Ishani, Nelson and Clothier21 Damage induced by subclinical or manifested episodes of AKI may produce irreversible loss of renal mass with deleterious effects on overall renal function. In follow-up studies of paediatric AKI patients, the incidence of CKD ranges from 27 to 67%. Reference Askenazi, Feig, Graham, Hui-Stickle and Goldstein20,Reference Shaw, Brocklebank, Dickinson, Wilson and Walker22
Mammen et al demonstrated a 10% incidence of CKD in 1–3 years after AKI in critically ill children (including post-operative cardiac patients), where almost half were at risk of chronic kidney disease. Reference Mammen, Al Abbas and Skippen23 However, there is no data available on whether children with AKI after the ASO are at increased risk of developing CKD. Greenberg et al demonstrated that 18% of children with perioperative AKI after paediatric cardiac surgery had chronic kidney disease at 5-year follow-up. Reference Greenberg, Zappitelli and Devarajan24 This is not specifically described in the literature for TGA after the ASO. Therefore, long-term follow-up with monitoring of renal function (serum creatinine and cystatin C), screening for hypertension and proteinuria is recommended, especially for children with AKI after paediatric cardiac surgery.
Study limitations
Children undergoing the ASO procedure within the first week of life may have elevated (maternal) creatinine levels preoperatively. Serum creatinine passes through the placenta, resulting in high levels at birth. During the first 72 hours of life, there is a difficulty in eliminating excess creatinine. Reference Muhari-Stark and Burckart25 Serum creatinine levels show a steady decline and reach stable levels by 1–2 weeks of age in full-term neonates. pRIFLE monitors the rise in creatinine levels to determine AKI in children aged 0–18. If the (maternal) creatinine is high before the operation, there could be an underestimation of the percentage rise and therefore an underestimation of the AKI group. For neonates, Several adjusted RIFLE scores have been proposed: some authors discarded urine output criteria. Reference Bezerra, Vaz Cunha and Libório26 Following previous similar studies with post-CPB surgery, we did not use the RIFLE urine output criteria in the patient classification as most children are oligo- or anuric in the first hours following CPB surgery. If urine output was used as a criterion for AKI, more children would have been scored as AKI. Baseline characteristics did show a significant difference in preoperative creatinine levels between severe AKI and non-AKI groups (i.e., lower in severe AKI group). Accordingly, because pRIFLE criteria are based on the percentage rise in creatinine, there is a certain risk of overestimating AKI in this population. However, baseline differences between the two groups (creatinine levels of 35 versus 41 mmol/L) were statistically significant but clinically not overly relevant.
Conclusion
Twenty-four percentage of the TGA patients treated with the ASO in our institution between 2000 and 2020 developed severe acute kidney injury in the post-operative period, according to pRIFLE criteria. This was associated with increased morbidity, longer PICU stay, and higher mortality. Long-term follow-up studies are required to assess whether children with severe AKI after the ASO are at higher risk of developing chronic kidney disease later in life.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (please name) and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees of the hospital.’








