Perinatal regionalization, known as tiered provision of neonatal care, emerged over time as a strategy to provide optimal, risk-appropriate maternal child services for a geographically dispersed population. These systems of regional perinatal services are now common across North America and Europe and are linked to improved outcomes of high-risk infants born either preterm or with serious medical or surgical conditions.Reference Bode, O’shea, Metzguer and Stiles 1 , Reference Clement 2 Following some initial recommendations, 3 the Committee on Fetus and Newborn issued two policy statements on Levels of Neonatal Care.Reference Stark 4 , 5 The 2012 policy statement recommended regionalized systems of perinatal care to ensure that each newborn infant is delivered and cared for in a facility most appropriate for his or her health care need. 5
Existing evidence links perinatal regionalization with improved neonatal outcomes for the infants born preterm and with low birth weight.Reference Lasswell, Barfield, Rochat and Blackmon 6 However, there are growing concerns about the high costs of neonatal intensive care as well as the substantial financial burden survivors of neonatal intensive care might pose to their families and the health care system.Reference Kilpatrick, Schlueter, Piecuch, Leonard, Rogido and Sola 7 , Reference Bohin, Draper and Field 8 Although the overall efficacy of specific neonatal intensive care interventions has been established in the literature,Reference Sinclair 9 , Reference Cifuentes, Bronstein, Phibbs, Phibbs, Schmitt and Carlo 10 limited evidence exists on the overall effectiveness of perinatal regionalization and its impact on long-term health outcomes.
This study evaluates the impact of perinatal regionalization on adverse long-term outcome based on data from the Canadian Multi-Regional Cerebral Palsy Registry (CCPR), which predated therapeutic hypothermia in Quebec. Our hypothesis was that the level of neonatal care available at the time of delivery may influence the risk of developing cerebral palsy (CP) non-ambulatory status. We thus sought to evaluate the association between the level of neonatal care available at delivery and CP nonambulatory status.
Materials and Methods
The study was conducted using Quebec provincial data from the CCPR, which predated therapeutic hypothermia within the Quebec perinatal-neonatal system. The description of this unique registry can be found in Oskoui et al.Reference Oskoui, Joseph, Dagenais and Shevell 11 Using the framework of the regionalization of pediatric rehabilitation service delivery, children with CP born in 1999 or after were enrolled within six of the province’s 17 administrative health regions capturing approximately half of the province’s population. Once cases are identified, parental consent is obtained and the maternal medical and obstetric records as well as the child’s neonatal, medical, and rehabilitation records are reviewed. These data are supplemented by a standardized parental interview and physical examination of the child by a pediatric neurologist, developmental pediatrician, or child physiatrist. For each enrolled child, more than 120 variables are collected and entered into a Research Electronic Data Capture database. Local ethics board approval was obtained from each participating institution. The Montreal Children’s Hospital-McGill University Health Center Research Institute ethics board provided central approval for data storage, analysis, and overall operations. To be enrolled in CCPR, a child must be at least 2 years of age and meet diagnostic criteria for CP, which include clinical diagnosis of a nonprogressive motor impairment resulting from a presumably early insult to the developing brain.Reference Rosenbaum, Paneth, Leviton, Goldstein, Bax and Damiano 12 A follow-up at 5 years of age is used to confirm the diagnosis and update functional outcome variables.
Children within the CCPR included for analysis in this study were born between 1999 and 2008 in the province of Quebec, allowing all to have a 5-year follow-up and confirmation of status. Children with CP diagnosis linked to any identified postneonatal cause or cases born outside the province of Quebec were excluded from this analysis.
For the analysis, we classified children according to level of neonatal care available where delivery was carried out. 5 In Quebec, maternity care is regionalized, and nearly all deliveries take place in public hospitals or birthing centers. Low-risk deliveries are carried out at level I hospitals (well newborn nurseries), level II hospitals (specialty care), or level III hospitals (subspecialty). We used clear, uniform definitions and consistent standards of level of neonatal care across Quebec, Canada, and appropriate adjustment for differences in case mix among the three groups of hospitals. We classified each delivery unit according to its level of neonatal care where delivery was carried out. 5 This classification reflects differences in the level of obstetric and neonatal competences available at the hospital and is outlined in more detail in Appendix 1.
The outcome used for this analysis was CP nonambulatory status, as defined by a Gross Motor Function Classification System level IV and level V.Reference Palisano, Rosenbaum, Walter, Russell, Wood and Galuppi 13 The challenge of this research question was to control for case mix differences. In particular, level II and level III hospitals have a higher proportion of medium- and high-risk pregnancies compared with level I hospitals. We used a quasi-experimental study design (with nonequivalent comparison groups pretest-posttest),Reference Shi 14 with controls for many relevant covariates to remove selection bias (bias by indication) that could originate from the differences in case mix among the three groups of hospitals. We used two standard methodologies to adjust for measured bias in these nonexperimental data. Methods included multivariate regression model risk adjustment and propensity-based matching. We first used multivariate linear regression, which allows for many risk factors to affect both referral to hospital type and outcome conditional on that referral. The multivariate linear regression model is the conventional modeling approach that incorporates all known confounders, including interactions, into the model. Controlling for these covariates produces a risk-adjusted treatment effect of the level of care and removes measured bias resulting from observed confounders. Second, we used propensity score matching, which is a specialized estimator that generally offers more precise estimates. However, we also used multivariate instrumental variable regression, which is a method designed to control for hidden bias in observational data. This technique allows for unobserved risk factors that affect the referral to hospital type and outcomes conditional on that referral, but which often suffers from imprecise estimates.
Our data contained a large number of variables about mother and child to make appropriate adjustments for differences in case mix between hospitals using propensity score matching. We used current clinical practice guidelines in obstetrics and gynecology, 15 perinatal surveillance literatureReference Liston, Sawchuck and Young 16 and CP risk factorsReference Trønnes, Wilcox, Lie, Markestad and Moster 17 to choose explanatory variables and make proper adjustments for differences in case mix between hospitals. The following covariates were used to control for risk factors (and deal with selection bias/bias by indication): preeclampsia, gestational diabetes, bleeding during pregnancy, severe illness during pregnancy, accident or trauma during pregnancy, preterm birth, a family history of CP, low maternal education (lacking a high school diploma), maternal age, history of drug use (We used current clinical practice guidelines in obstetrics and gynecology, 15 perinatal surveillance literature,Reference Liston, Sawchuck and Young 16 and CP risk factorsReference Trønnes, Wilcox, Lie, Markestad and Moster 17 to choose these control variables.). We also controlled for perinatal asphyxia, which was defined as neonatal encephalopathy with at least three of the following criteria: an Apgar score <6 at 5 minutes, a cord pH <7.0, a cord base excess >12, an abnormal fetal heart rate such as tachycardia (>160 beats per minute) or bradycardia (<120 beats per minute), presence of meconium, need for intubation, delay in spontaneous respiration, need for resuscitation of the newborn, multisystem involvement in the neonatal period, or abnormal imaging results consistent with hypoxic ischemic injury. We also tested for effect modification between perinatal asphyxia and level of care at delivery. However, the interaction variable was not statistically significant and postestimation tests suggested that it did not statistically significantly improve the model’s performance. We subsequently did not include this interaction term.
Results
Our cohort of 360 children with CP without any postneonatal cause was born in Quebec between 1999 and 2008. Forty-six percent were born in birth sites with level III neonatal care, 20% with level II, and the remainder (34%) with level I neonatal care. Nonambulatory status (Gross Motor Function Classification System levels IV and V) was reported in 27%. The other characteristics of the population are presented in Table 1.
Table 1 General characteristics of the population

CP=cerebral palsy; GMFCS=Gross Motor Function Classification System; SD=standard deviation
Propensity score matching estimates, standard errors and associated 95% confidence intervals are displayed in Table 2. These estimates measure risk-adjusted change in the probability of nonambulatory CP status because of increased level of service at delivery. Propensity score matching generally pointed to no effect relationship between the level of neonatal care at delivery where birth was carried out and later CP nonambulatory status. There was no statistical evidence that delivery carried out in levels II or III hospitals versus level I hospital has any effect on the incidence of CP nonambulatory status. We found risk estimates for level II versus level I and level III versus level I be weakly negative and not statistically significant. A positive and statistically significant risk estimate was found for the level III versus level II comparison. However, the propensity score matching substantially reduced the case mix differences between the groups of hospitals as absolute standardized bias after adjustments was <5% for most risk factors. Our instrumental variables methods estimation confirmed no effect relationship between the level of care available at time of delivery and the CP nonambulatory status (Appendixes 3-5). Endogenous bivariate probit estimates identified no effect relationship with highest precision. We found that CP nonambulatory status was not affected by the type of hospital where delivery was carried out across propensity score matching and instrumental variables methods.
Table 2 Propensity score matching results
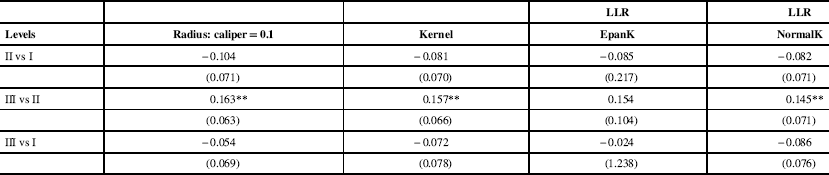
Propensity score estimates measure risk-adjusted change in the probability of non-ambulatory CP status resulting from increased level of service at delivery. Bootstrapped standard errors in parentheses (300 repetitions).
EpanK=Epanechnikov kernel; LLR=local linear regression; NormalK=Gaussian kernel.
Linear probability model did show that several point estimates were statistically significant at conventional levels and are noteworthy (Table 3). These models found a statistically significant association between CP nonambulatory status and perinatal asphyxia (p<0.01), low maternal education (p<0.1), and preterm birth (p<0.1).
Table 3 Linear probability model results

Level I hospitals are the base category. Robust standard errors are in parentheses. Statistical power=0.76. A 10% significance level is chosen because there is very little knowledge and no firm belief regarding the null hypothesis based on past experience.
Asphyxia was present in 15% of these children; 15.5% were born at sites with level I neonatal care, 18.6% were born at sites with level II neonatal care and 12.6% at sites with level III neonatal care. We find that asphyxiated versus nonasphyxiated kids have 2.86 (95% confidence interval, 1.57-5.21) the unadjusted odds of developing a CP nonambulatory status, whereas linear probability model suggests that presence of perinatal asphyxia increases the probability of nonambulatory status by 27% all else being equal. Preterm birth is found to increase the chances of later CP nonambulatory status by 9% all else equal, and low maternal education increased the risk of CP nonambulatory status by 2%.
Discussion
In Quebec, maternity care is regionalized, and nearly all deliveries take place in public hospitals. Low-risk deliveries are carried out at level I hospitals including birthing centers, whereas medium- and high-risk deliveries are referred to levels II or III hospitals. Prediction of the infant’s state at birth determines referrals to level I, II, or III hospitals. High-risk deliveries are identified based on unified national medical guidelines. 15 , Reference Liston, Sawchuck and Young 16 , Reference Gilbert, Fell, Joseph, Liu, León and Sauve 18 Our study demonstrated that the majority of children developing CP for perinatal reasons were born in birth sites with level III neonatal care. It reflects that, despite the current high level of technology and obstetric and neonatal competences, outcome of the high-risk deliveries, referred to birth sites with level III neonatal care, still leads to significant long-term complications. Interestingly, 34% of children developing CP for perinatal reasons were born in sites with level I neonatal care.
We have shown that the differences in the level of neonatal care at the time of delivery did not seem associated with the risk of CP nonambulatory status. Our finding is consistent and robust across methods and empirical specifications used. Propensity score matching models suggested no effect relationship between the level of neonatal care available at hospital where delivery was carried out and risk of CP nonambulatory status between level II versus level I, level III versus level I comparisons. Positive coefficients were evident across the level III versus level II comparison, suggesting level II centers as protective; however, this might be reflective of the propensity score method inability to eliminate selection effects or unobserved heterogeneity between the two groups of hospitals. Instrumental variables estimation allowed us to control for possible selection effects and consistently found no relationship between the level of neonatal care at hospital where delivery was carried out and CP non-ambulatory status. To our knowledge, this is the first study where case mix adjustment was used to study the effects of perinatal regionalization on long-term outcomes and using instrumental variables methods.
The lack of impact of the level of neonatal care at the time of delivery on the risk of CP nonambulatory status is probably demonstrating the benefit of the development and generalization of the neonatal resuscitation program. The neonatal resuscitation program is for North American health care providers working in the delivery room and nurseries and is designed to aid in learning the cognitive and technical skills required for resuscitation of the newborn born babies and referral to specialized centers as soon as possible. 19 , Reference Kattwinkel 20 Neonatal resuscitation was shown to reduce mortality from intrapartum-related events,Reference Bang, Bang, Baitule, Reddy and Deshmukh 21 - Reference Lee, Cousens, Wall, Niermeyer, Darmstadt and Carlo 23 such as perinatal asphyxia, and might explain no effect relationship found in our study. However, the lack of relationship between CP nonambulatory status and level of service at delivery might be due to the fact that within Quebec regionalized maternity service high-risk deliveries are identified in advance and are subsequently referred to hospital with appropriate level of service. This finding is consistent with exiting evidence that a regionalized maternity service within a publically owned and financed health system does not lead to increased infant morbidity.Reference Grytten, Monkerud, Skau and Sørensen 24
Perinatal asphyxia was highlighted as a significant risk factor for CP nonambulatory status because asphyxiated versus nonasphyxiated kids have 2.86 the odds of developing a CP nonambulatory status. In our cohort, the proportion of children with perinatal asphyxia born in each of the three neonatal care levels were approximately equal, being 15.5%, 18.6%, and 12.6% in levels I, II, and III, respectively. However, nonambulatory status was more unevenly distributed and was present in 28.5% of kids born in level I, 20.0% of kids born in level II, and 29.3% of kids born in deliveries with level III. Perinatal asphyxia cannot be predicted before birth and thus has to be managed acutely in hospitals with different levels of technology and obstetric and neonatal competences available for neonatal care. Type of therapy the child receives immediately after birth is an important determinant of later CP severity. Newborns with perinatal asphyxia born in level I hospitals are usually transferred to level II or III hospitals, which have the capacity to provide an increased level of further care such therapeutic hypothermia. It is important to note that therapeutic hypothermia became widely available in Quebec as of 2009, so the birth cohorts included in this study did not have this therapeutic option. Lack of recognition of the patients that could benefit from this treatment remains a challenge, however, because there is a narrow window of opportunity to act. Because the proportion of births complicated by perinatal asphyxia among children with CP was evenly distributed among levels of neonatal care available at birth sites, it would be of interest to study the impact of lack of local availability of therapeutic hypothermia in level I centers on later risk of nonambulatory status. Our results also indicate that preterm birth and low maternal education may constitute important independent risk factors for CP nonambulatory status, which deserve further study.
Our study has several limitations. Methods used cannot replace a randomized controlled trial and thus might not be able to fully control for selection effects or unobserved covariates. However, a randomized controlled trial that would assess the impact of levels of neonatal care available at hospitals where delivery was carried out on CP ambulatory status is likely not to be undertaken given pragmatic concerns. Mortality is a competing outcome because it could potentially have reduced the number of CP nonambulatory cases. However, in our analysis, we observe mortality outcomes and we coded deaths as CP nonambulatory cases. Our rich data do not allow us to control for all possible postneonatal factors including the regional variability of pediatric rehabilitation services We find that differences in the level of care at delivery are not associated with CP nonambulatory status; however, our study does not discusses differences in other measures that might be important as well for determination of outcomes of regionalized perinatal care such as fine motor skills, cognition, language, or behavior.
In conclusion, our study implies that a regionalized maternity service does not affect the distribution of CP nonambulatory cases. This suggests that level of care and associated medical technology within the Quebec regionalized neonatal-perinatal system, is used efficiently because it does not offer any further marginal benefit in the reduction of severe CP outcomes. The system works well as it is and this is supportive of the perinatal regionalization. The success of the neonatal resuscitation program and referral of high-risk births to regional hospitals with sufficient obstetric and perinatal competence and resources may contribute to this lack of variability. Further research is needed to understand the causal links and associated mechanisms between prenatal risk factors, perinatal asphyxia, and CP severity.
Disclosures
Pia Wintermark has no disclosures relevant to this article. Michael Shevell and Maryam Oskoui have received research funding from NeuroDevNet for work in cerebral palsy. Maryam Oskoui has received research funding from CIHR for work in cerebral palsy. Corneliu Bolbocean received funding from NeuroDevNet Doctoral Fellow and Open Society Foundations GSGP Grant.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/cjn.2015.322





