Introduction
Hemangiopericytoma (HPC) is a rare, aggressive, and highly vascularized mesenchymal tumor. Many features of HPC resemble meningiomas,Reference Tashjian, Khanlou and Vinters1–Reference Mena, Ribas and Pezeshkpour4 which explains why these were initially classified as angioblastic meningiomas by Cushing and Eisenhardt.Reference Bailey, Cushing and Eisenhardt5 HPCs are derived from fibro-histiocytic precursor cells, the pericytes of Zimmerman.Reference Laviv, Thomas and Kasper6 These are immature spindle cells with contractile properties that attach to capillary walls.Reference Melone, D’Elia and Santoro7 The cells of Zimmerman are crucial in mechanically supporting the capillaries, aiding thus in luminal size changes to different physiological challanges.Reference Bose8,Reference Ben Nsir, Badri and Kassar9 HPC is a systemic neoplasm, frequently involving the skin and musculoskeletal system.Reference Ben Nsir, Badri and Kassar9 Intracranial cavity involvement is rare in HPC, constituting approximately 0.4% of all intracranial lesions and 2.4% of meningeal lesions.Reference Guthrie, Ebersold and Scheithauer3,Reference Melone, D’Elia and Santoro7,Reference Cohen-Inbar, Lee and Mousavi10,Reference Sonabend, Zacharia and Goldstein11 The first report of an intracranial HPC can be dated to 1954, by Begg and Garret,Reference Begg and Garret12 but these were classified as a distinct pathological entity by the World Health Organization (WHO) only in 1993, based on clinical, immunohistochemical, ultrastructural, and genetic features.Reference Giannini, Rushing, Hainfellner, Louis, Ohgaki, Wiestler and Cavenee13–Reference Ramsey15 The 2016 WHO classification defines the solitary fibrous tumors (SFTs)–HPC entities on a single spectrum with a single grading system. This is based on unique genetic events occurring in these pathologies, that is, the fusion of NAB2 and STAT6 genes.Reference Louis, Perry and Reifenberger16 In addition, histological parameters of proliferation (MIB-1 index)Reference Galanis, Buckner and Scheithauer17,Reference Vuorinen, Sallinen and Haapasalo18 and classical features of malignancy (cellular atypia, necrosis, mitotic figures, etc.)Reference Mena, Ribas and Pezeshkpour4 are known negative prognostic factors in SFT-HPCs. HPCs have a slight male predominance,Reference Mena, Ribas and Pezeshkpour4 and a mean age of 38–42 years at presentation. Most intracranial HPCs are supratentorial (62%).Reference Laviv, Thomas and Kasper6,Reference Jaaskelainen, Servo and Haltia19,Reference Schroder, Firsching and Kochanek20
HPCs are notorious for their “aggressive” biology, featuring high recurrence rates (reaching 91% after surgical resection in some reports),Reference Vuorinen, Sallinen and Haapasalo18 distant intracranial and neural axis metastasis (DNM), and extraneural metastasis (ENM), appearing even after a gross-total resection (GTR) was achieved. The cumulative risk of ENM reaches as high as 70% in 15 years.Reference Goellner, Laws and Soule2–Reference Mena, Ribas and Pezeshkpour4,Reference Laviv, Thomas and Kasper6,Reference Cohen-Inbar, Lee and Mousavi10,Reference Chan, Cheuk and Ho21–Reference Ghia, Chang and Allen26 The incidence of both DNM and ENM increases with time, serving as a negative prognostic factor.Reference Kano, Niranjan and Kondziolka27
Treatment of HPC is multidisciplinary and challenging. Micro-surgical resection, when feasible, still serves as the initial treatment of choice for large HPCs.Reference Sheehan, Kondziolka and Flickinger28 Microsurgical resection offers several benefits for intracranial HPC, including the immediate relief in the clinical manifestations of related mass effect, as well as provides tissue diagnosis and characterization. Yet, the florish “staghorn” vascular nature of HPCs and frequent involvement of adjacent meningeal dural venous sinuses and cranial osseous components can make surgical GTR a formidable and at times unrealistic goal.Reference Cohen-Inbar, Lee and Mousavi10 Most long-term follow-up reports note that the majority of HPC patients require a multidisciplinary, doctrine crossing, approach with different modalities serving as “salvage” or “complimentary” to prevent recurrence or progression of the HPC disease.Reference Cohen-Inbar, Lee and Mousavi10,Reference Sonabend, Zacharia and Goldstein11,Reference Ghia, Chang and Allen26–Reference Rutkowski, Sughrue and Kane29 We present a short two-part discussion on central nervous system (CNS) HPCs, reviewing current treatment paradigms. In Part I, we focus our discussion on the challenges of intracranial HPC.
Imaging Features
Cranial Hemangiopericytomas
The radiographic differentiation of intracranial HPCs from a meningioma is pivotal in the preoperative management planning and choice of surgical approach, owing to the higher risk of severe bleeding and of local recurrence, even after a “conventional” Simpson grade-I GTR.Reference Schirmer and Heilman30 Radiographic similarities to meningiomas are profound, and distinguishing HPCs can be challenging. HPCs typically feature lobulated margins, frequent internal serpentine flow-related signal voids, and absent calcifications. This is different from meningiomas, which typically have smooth margins, no flow voids, and abundant calcifications (20–25%).Reference Sibtain, Butt and Connor31 HPCs typically feature a paucity of peritumoral edema and a unique distinct angio-architectural pattern. This pattern involves a dual blood supply from intracranial and extracranial blood vessels. Unlike in meningiomas, the dominant blood supply in HPCs typically arises from the internal carotid artery (ICA) or vertebral artery (VA) branches [rather than the external carotid artery (ECA) in meningiomas] which manifests in numerous corkscrew vessels seen arising from a main arterial feeder within the tumor on digital substraction angiography (DSA).Reference Schirmer and Heilman30 This DSA feature results in a long-lasting contrast enhancement pattern, compared to the typical sunburst ECA-related DSA pattern seen in meningiomas.Reference Marc, Takei and Schechter32
The MRI appearance of intracranial HPCs may be similar to that of meningiomas. Unique HPC-related features include a narrow base of attachment, an irregular/multilobulated cross-leaf growth, the absence of intratumoral calcifications, the absence of related osseous hyperostosis,Reference Zhou, Liu and Zhang33 bone erosion, and heterogeneous gadolinium contrast enhancement.Reference Liu, Chen and Ma34 Cranial HPCs are typically isointense with gray matter on both T1- and T2-weighted MRI sequences. Mixed signals when noted were shown to be associated with the grade-III (anaplastic) aggressive HPC type.Reference Zhou, Liu and Zhang33 A “dural tail” is seen in 30% of grade-II patients.Reference Liu, Chen and Ma34 The grade-III anaplastic HPC variant is marked by the presence of necrosis, cystic changes, and extensive peritumoral edema.Reference Zhou, Liu and Zhang33 Diffusion-weighted imaging in grade-III HPCs is marked by higher ADC values compared to grade-II HPCs, meningioma, or with the normal surrounding brain.Reference Liu, Chen and Ma34,Reference Liu, Yin and Geng35 Whole-tumor histogram analysis of ADC maps may be a useful tool for differential diagnosis, with ADCmin and ADC5 being potential parameters.Reference He, Xiao and Li36
Spinal Hemangiopericytomas
Spinal HPCs can be divided into intradural (ID) and extradural (ED) lesions. The ID HPCs in turn, can be intramedullary (IM) or extramedullary (EM).Reference Merhemic, Stosic-Opincal and Thurnher37 The ED HPCs are further classified as either dural-based or primarily osseous.Reference Ramdasi, Nadkarni and Goel38 These distinctions, maybe in spinal HPCs more than cranial HPCs, have a dramatic and significant influence on the prospect of attaining a GTR, risk of neurological morbidity, recurrence, and so on. Radiographic features of spinal HPCs include a multilobular or “dumbbell”-shaped mass, expanding and eroding adjacent vertebral cortical bone. These lesions are typically hypointense on T1WI, moderately hyperintense on T2WI, with an homogeneous gadolinium enhancement pattern, at times with internal vessel voids (similarly to the cranial HPCs).Reference Zhang, Hu and Zhou39–Reference Patnaik, Jyotsnarani and Uppin41 Advanced dynamic MRI-imaging techniques, such as diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI), have been employed in the evaluation of spinal lesions.Reference Landi, Palmarini and D’Elia40 DTI and fiber tractography analyses allow for a better preoperative diagnosis. A better understanding of the patient’s altered white matter microanatomy and tracts in relation to the lesion is provided (for ID-IM and ID-EM HPCs), more so than a conventional MRI study, in planning the surgical intervention and counseling the patient on potential risks and accepted post-operative morbidity.Reference Alkherayf, Arab and Tsai42
Computed tomography (CT) and myelography are less frequently utilized modalities nowadays for ID-IM or ID-EM HPCs, yet may still have a role in assessing the osseous components and the need for spinal stabilization upon resection (ED HPCs or those spanning both compartments). Spinal positron emission tomography/CT using either fludeoxyglucose or 11(C)-methionine has shown efficacy and a potential role in the evaluation of high-grade malignant ID-IM lesions.Reference Naito, Yamagata and Arima43 Protoporphyrin IX (PpIX) fluorescence induced by 5-aminolevulinic acid (5-ALA) was recently shown relevant and helpful in the detection of potential spinal-HPC tumor residual during microsurgical resection.Reference Millesi, Kiesel and Woehrer44
Multimodal Management
The management of intracranial HPCs, being a rare entity, is not referred to in the National Comprehensive Cancer Network Clinical Practice Guidelines.45 Clinical standard guidelines are thus lacking, and treatment plans are typically based on single institution retrospective small series,Reference Fountas, Kapsalaki and Kassam46,Reference Olson, Yen and Schlesinger47 multicenter small cohorts, and Surveillance, Epidemiology and End Results (SEER) analyses.Reference Hall, Ali and Gullett48–Reference Stessin, Sison and Nieto50 Multimodal treatment consisting of GTR (when feasible) and external beam radiotherapy (EBRT) is considered standard care, noted to convey an average survival of 84 months from the time of diagnosis, not accounting for neurological and overall morbidity.Reference Kumar and Wani51 The recurrence rates after GTR and EBRT are reported to be high, reaching 30%.Reference Dufour, Metellus and Fuentes25 The literature on spinal HPCs is composed mainly of case reports or short case series with approximately 112 cases of primary spinal HPCs published to date (all locations). A comprehensive review of the literature is shown in Table 1.Reference Schirger, Uihlein and Parker52–Reference Sweid, Noureldine and Nasser96 In addition, the significant incidence of operative neurological morbidity, coupled with the high recurrence and progression rates, optimal management is still a relevant open question.
Table 1: Hemangiopericytoma of the spine literature review
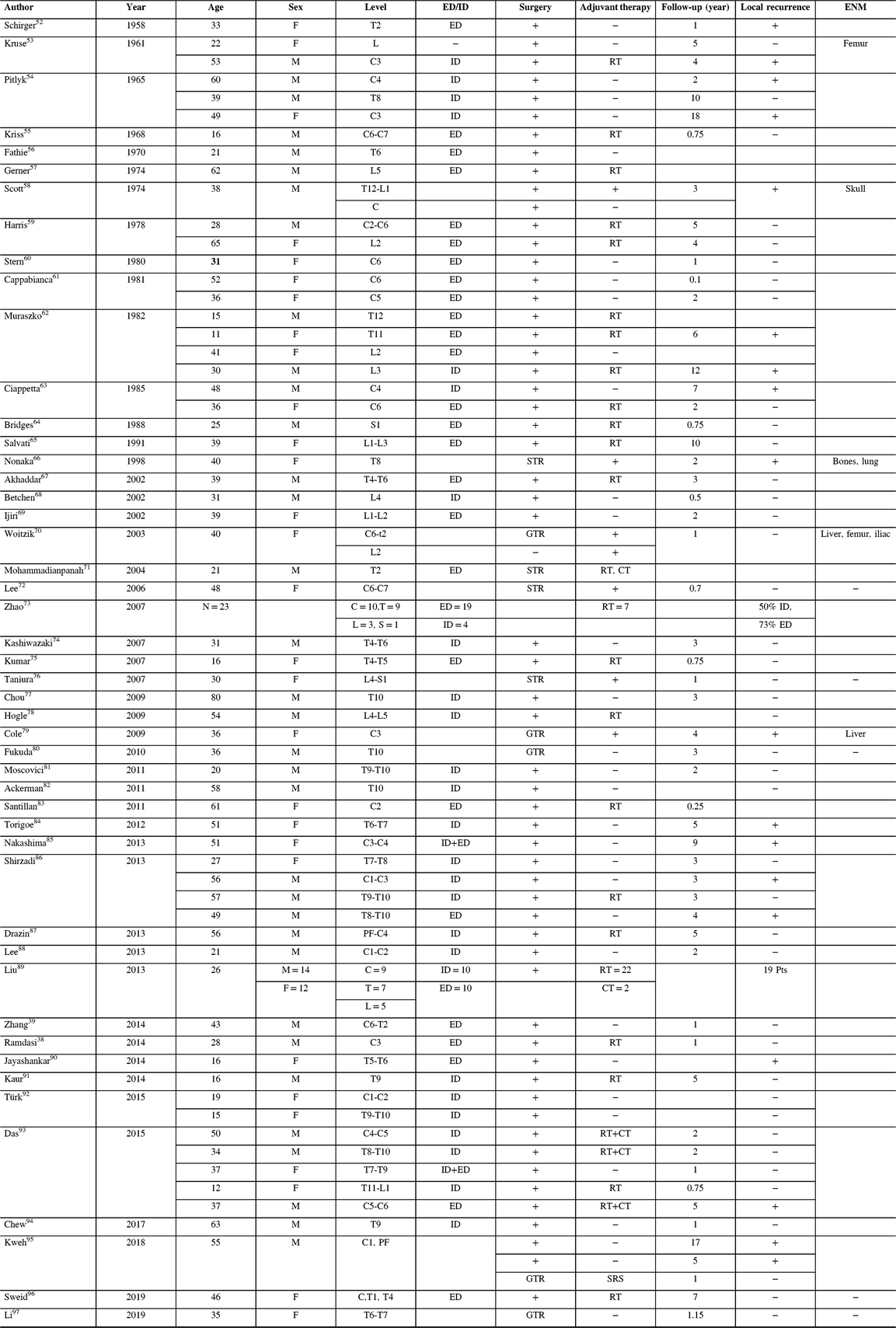
HPC = hemangiopericytoma; ENM = extraneural metastasis; ED/ID = extradural/intradural; RT = radiotherapy; SRS = stereotactic radiosurgery; M = male; F = female; GTR = gross total resection; STR = subtotal resection; D = dead from disease; A = alive with disease; PF = posterior fossa; CT = chemotherapy; L = lumbar; T = thoracic; C = cervical; S = sacral.
The clinical presentation of spinal HPC is nonspecific, depending mainly on lesion location, lesion size, the main compartment involved (ID vs. ED), the extent of spinal cord, and nerve root compression or involvement, not differing with various HPC histologic grades.Reference Li, Deng and Li97 Neurological deficits and weakness are common for ED HPCs owing to the diminishing ED compartment, similarly to other spinal ID lesion types.Reference Dufour, Metellus and Fuentes25,Reference Betchen, Schwartz and Black68,Reference Shirzadi, Drazin and Gates86,Reference Ecker, Marsh and Pollock98 Primary osseous spinal HPCs conversely typically manifest with pain and mass effect, owing to the lesions expending into the paravertebral gutter region and cortical bone.Reference Liu, Cao and Liu99
Formulating an optimal hybrid treatment approach to recurrence and progression is another such unmet challenge. Adjuvant and neo-adjuvant chemotherapy,Reference Olson, Yen and Schlesinger47,Reference Beadle and Hillcoat100 immunotherapy, or endovascular embolization has shown limited benefit.Reference Galanis, Buckner and Scheithauer17,Reference Beadle and Hillcoat100 We will shortly discuss each treatment modality in the battle against intracranial HPCs.
Microsurgical Resection
Cranial Hemangiopericytomas
Microsurgical resection offers the benefits of attaining a definite histopathological tissue diagnosis confirmation and analysis coupled with immediate alleviation of tumor-related mass effect. Achieving a GTR at the first operation was shown to be strongly associated with the prolongation of overall survival (OS) and progression-free survival (PFS) in intracranial HPC.Reference Guthrie, Ebersold and Scheithauer3,Reference Cohen-Inbar, Lee and Mousavi10,Reference Galanis, Buckner and Scheithauer17,Reference Dufour, Metellus and Fuentes25,Reference Rutkowski, Sughrue and Kane29,Reference Fountas, Kapsalaki and Kassam46,Reference Kim, Jung and Kim101,Reference Soyuer, Chang and Selek102 Still, microsurgical resection has been shown to provide poor long-term control of intracranial disease when employed solely, despite being considered as the treatment of choice.Reference Goellner, Laws and Soule2,Reference Guthrie, Ebersold and Scheithauer3,Reference Cohen-Inbar, Lee and Mousavi10,Reference Sheehan, Kondziolka and Flickinger28 In many cases, tumor location and neuroanatomical features may not allow a GTR. HPC of the skull-base not allowing dural resection, lesions involving the cavernous sinus or other dural venous sinuses not allowing tumor GTR with margins, HPC encroaching or casting critical neurovascular structures such as cranial nerves, major arterial branches are a few such limitations. The staggering reported surgical mortality rates for these lesions reach as high as 9–24%, much higher when neurological operative morbidity is reviewed and factored in.Reference Cohen-Inbar, Lee and Mousavi10,Reference Jaaskelainen, Servo and Haltia19,Reference Kano, Niranjan and Kondziolka27,Reference Sheehan, Kondziolka and Flickinger28,Reference Payne, Prasad and Steiner103 Such figures are considered unacceptable in present-day neurosurgery and neuro-oncology practices, which resulted in many surgeons dithering from attaining a GTR in such cases. Of note, even when GTR is attained, curing HPC is challenging with surgery alone due to HPCs’ propensity for recurrence, DNM, and ENM.Reference Kim, Kong and Seol104
Rutkowski et al.Reference Rutkowski, Sughrue and Kane29 reported a large intracranial HPC’s cohort of 563 patients in 2010, trying to define important prognostic factors affecting mortality. Overall median survival reported was 13 years, with a 1-, 5-, 10-, and 20-year survival rates of 95%, 82%, 60%, and 23%, respectively.Reference Rutkowski, Sughrue and Kane29 GTR achieved in surgery was noted to correlate with a median survival of 13 years, compared to the STR cohort having a median survival of 9.75 years. A complete, extended GTR (Simpson Grade I resection) was noted to be associated with improved PFS. STR was shown to correlate with high recurrence/progression rates (approaching 100%).Reference Fountas, Kapsalaki and Kassam46,Reference Bassiouni, Asgari and Hübschen105 The median time to local recurrence was reported to range 12–96 months with higher numbers (lower recurrence rates) in studies employing a multimodality treatment approach.Reference Galanis, Buckner and Scheithauer17,Reference Ghia, Chang and Allen26 Multiple microsurgical resections are feasible on occasion, yet the summated appreciable morbidity associated with each resection makes this option less attractive to both surgeons and patients.Reference Cohen-Inbar, Lee and Mousavi10
Melone et al.Reference Melone, D’Elia and Santoro7 reported a single center–based cohort of 36 patients with HPCs in 2014, with all initially undergoing a microsurgical resection. At a median follow-up duration of 118 months, the median OS was 84 months. The actuarial survival rates at 5 and 10 years were 94% and 72%, respectively. GTR was reported in 70% of patients at the initial surgery, and adjuvant EBRT was administered to 37% of GTR and 78% of STR patients. Patients who received STR were treated with SRS (50%) or proton beam therapy (50%) as well. Patients who underwent GTR had significant longer OS and PFS as compared to patients who underwent a STR (p = 0.047 and p = 0.0025, respectively).Reference Melone, D’Elia and Santoro7 The reported local recurrence rates range 26–80%, depending on multiple factors, such as the extent of resection (GTR vs. STR) and length of follow-up, adjuvant RT/SRS.Reference Goellner, Laws and Soule2–Reference Mena, Ribas and Pezeshkpour4,Reference Cohen-Inbar, Lee and Mousavi10,Reference Kano, Niranjan and Kondziolka27 Dufour et al. reported in 2001 a very high 88% recurrence rate after microsurgical resection alone. This figure dropped to 12.5% with the addition of EBRT.Reference Dufour, Metellus and Fuentes25 Guthrie et al. reported that the addition of adjuvant EBRT after resection increased PFS and OS from 34 and 62 months to 75 and 92 months, respectively.Reference Guthrie, Ebersold and Scheithauer3 Adjuvant EBRT or SRS after a GTR was not shown to improve OS significantly, yet still improved local tumor control rates.Reference Ghia, Chang and Allen26,Reference Sheehan, Kondziolka and Flickinger28,Reference Olson, Yen and Schlesinger47,Reference Stessin, Sison and Nieto50 Other reports did not show any improvement in either OS or PFS.Reference Rutkowski, Jian and Bloch22,Reference Ecker, Marsh and Pollock98,Reference Kim, Kim and Chung106
The current literature is still conflicted as to what degree the extent of resection correlates with the rate of recurrence, incidence of DNM, or response to adjuvant treatment.Reference Olson, Yen and Schlesinger47 It seems that a micro-surgical resection should ideally be carried out to the point of maximal safe reduction in tumor volume, while preserving neurological function. Modern treatment approaches employ the adoptive hybrid surgery (AHS) approach (planned subtotal resection followed with interval planned SRS), which allows for the preoperative planning of the extent of resection, the irradiated target volume, and other related parameters.Reference Cohen-Inbar and Sviri107
Spinal Hemangiopericytomas
Li et al.Reference Li, Deng and Li97 recently reported a cohort of 94 patients operated for spinal HPC. In this report, an alarming and staggering overall 50% recurrence rate was noted. Recurrence was highest in HPCs spanning both compartments (ID + ED HPCs), reaching as high as 75%. Isolated ID HPCs had a lower yet still significant 38.5% local recurrence, and isolated ED had a 44.8% recurrence rates after a seemingly GTR.Reference Li, Deng and Li97 Some authors advocate for an extended GTR approach (entailing resection of the peri-lesional neighboring dura as well) for spinal HPCs in order to prevent local recurrence of metastases.Reference Olson, Yen and Schlesinger47,Reference Ijiri, Yuasa and Yone69,Reference Nakashima, Imagama and Sakai85,Reference Beadle and Hillcoat100–Reference Soyuer, Chang and Selek102 Still, other authors report a much less significant role for GTR. Liu et al.Reference Liu, Yang and Chen89 reported in 2013 a series of 26 patients operated for spinal HPCs in which GTR did not influence OS or PFS.Reference Liu, Yang and Chen89
It is misleading, to some extent, to discuss operative outcome in all-locations spinal HPC together. Lumping tumors in these different compartments together results in a negative bias toward “simpler” lesions. Surgical morbidity for a small osseous, vertebral body-centered ED-HPC is very different clinically and surgically from a large ID-IM HPC. Preoperative electrophysiological evaluation and functional imaging studies as well as intra-operative electrophysiological monitoring are crucial and indispensable. No such surgical endeavor should be undertaken without these measures. Thus, a GTR can be attempted for spinal ED-HPC or ID-EM HPC lesions having clear margins, serving to relieve tumor-related mass effect and neurological morbidity related to direct pressure. An AHSReference Cohen-Inbar and Sviri107 approach seems prudent for lesions enveloping functional nerve roots or vascular structures (ID-EM or ID-IM). In this approach a planned STR is followed with SRS or RT, reported to reduce recurrence rates and improve OS in spinal HPCs.Reference Jaaskelainen, Servo and Haltia19,Reference Dufour, Metellus and Fuentes25,Reference Chacko, Chacko and Rajshekhar108,Reference Tso, Wang and Yang109
Chemotherapy/Immunotherapy/Embolization
Many chemotherapeutic agents and combinations have been tested, yet an effective drug regimen against HPC is still lacking.Reference Mathieu110 Chamberlain et al. reported in 2008 the use of sequential multiple drug regimens in a cohort of 15 patients with recurrent HPCs who received adjuvant EBRT.Reference Kruse53 In their report, first line drugs consisted of cyclophosphamide, doxorubicine, and vincristine (CAV). Second-tier drugs were alpha-interferon and then ifosfamide, cisplatin, and etoposide (ICE), in case of subsequent recurrence/failure. A few temporary responses were noted with the CAV regimen, and the OS was 14 months.Reference Chamberlain and Glantz111 Pathologic studies on resected HPC specimens allowed PierscianekReference Pierscianek, Michel and Hindy112 to demonstrate upregulation of several key signaling pathway markers, including VEGF-VEGFR 2, EphrinB2-EphB4, and DLL4-Notch. Reported in 2016, findings were not different between HPC grade-II or grade-III. These markers may serve as potential targets for therapy, especially considering the known vascular nature of this tumor.
Angiogenic pathway studies paved the way to initial testing of bevacizumab, a monoclonal anti-VEGFR antibody commonly used for the treatment of colorectal cancer and recurrent glioblastoma in intracranial HPC.Reference Pierscianek, Michel and Hindy112 Initial studies show activity against HPCs when administered aloneReference Rutkowski, Sughrue and Kane29,Reference Michishita, Uto and Nakazawa113–Reference Sun, Liu and Wang115 or in combination with temozolomide.Reference Park and Araujo116 Tyrosine kinase inhibitors targeting either EphA2 or EphB4 are another potential therapeutic venue.Reference Barquilla and Pasquale117
Preoperative embolization of tumor-related feeding vessels (similar to common practice in meningioma surgery, embolizing ECA vessels) can prove helpful in controlling operative bleeding. Still, such preoperative embolization plays a limited role in intracranial HPC management due to these tumors’ tendency to parasitize and invade feeding cortical vessels from both the ECA and ICA. Such angioarchitecture does not allow asymptomatic vessel sacrifice.Reference Cohen-Inbar, Lee and Mousavi10,Reference Olson, Yen and Schlesinger47
External Beam Radiotherapy
The use of EBRT in HPCs postoperatively is widely accepted due to high recurrence rates after surgery alone.Reference Chang and Sakamoto24 The first application of EBRT for HPCs can be traced back to a report from 1974 by Dube and Paulson, where the authors reported a complete tumor response.Reference Dube and Paulson118 Mira et al. reported in 1977 a short series of 11 HPC patients treated with over 29 courses of EBRT, noting a positive clinical response in 26 of 29 courses.Reference Mira, Chu and Fortner119 EBRT was reported to decrease local recurrence rates to 12.5% after a fractionated dose of 50–64 Gy. These figures contrast an 88% recurrence rate after GTR alone.Reference Dufour, Metellus and Fuentes25 Some authors reported a role of EBRT as neoadjuvant therapeutic approach as well, namely prior to resection,Reference Uemura, Kuratsu and Hamada120 presumably by reducing tumor vascularity thus allowing for a safer surgical extirapation.Reference Uemura, Kuratsu and Hamada120 Multiple reports established that the response of HPCs to RT (different schedules) is dose dependent.Reference Schirmer and Heilman30 Total treatment doses of at least 45 Gy were shown to result in significantly superior local control rates.Reference Chang and Sakamoto24 Current common schedules deliver a total of 45–52 Gy doses over 25–35 fractions. SRT (a hybrid technique halfway between EBRT and SRS discussed later) can also serve as a potent salvage strategy for the treatment of recurrent intracranial HPCs.Reference Guthrie, Ebersold and Scheithauer3,Reference Dufour, Metellus and Fuentes25,Reference Olson, Yen and Schlesinger47 Unlike the stereotactic-focused techniques mentioned, EBRT has a leading role in treating patients with a diffuse HPC disease and widespread cranial/brain involvement.
The role of EBRT in the management of spinal HPC is controversial as well, and opinions vary. Chou et al.Reference Chou, Hsu and Lin77 reported in 2009 on a series of 16 patients with spinal HPCs who received adjuvant EBRT. In this cohort, EBRT had no effect on any of the different outcome parameters measured, mainly local recurrence. The histopathologic grade was the only prognostic factor deemed significant for recurrence.Reference Chou, Hsu and Lin77 This is a very small cohort, and any statistical analyses-based conclusions should be taken with a grain of salt (or skepticism), yet similar conclusions were drawn by other groups such as Liu et al.Reference Liu, Yang and Chen89 or Payne et al.Reference Payne, Prasad and Steiner103 in 2000.
Stereotactic Radiosurgery
The role of SRS in treating various malignant and metastatic tumors is undeniable.Reference Sheehan, Niranjan and Flickinger121 HPCs share different traits making this lesion well suited for SRS, such as well-defined margins from clear radiographic delineation, the potential for residual and recurrent tumor,Reference Tashjian, Khanlou and Vinters1 surgically challenging intracranial location (adjacent to crucial structures), and small volume lesions in unreachable locations.Reference Chang and Sakamoto24 Treating HPCs with SRS [with either a Gamma Knife system (Elekta AB) or CyberKnife (Accuray)] has been described extensively, with reported tumor control rates ranging 46–100%.Reference Cohen-Inbar, Lee and Mousavi10,Reference Coffey, Cascino and Shaw14,Reference Galanis, Buckner and Scheithauer17,Reference Chang and Sakamoto24,Reference Dufour, Metellus and Fuentes25,Reference Sheehan, Kondziolka and Flickinger28,Reference Olson, Yen and Schlesinger47,Reference Ecker, Marsh and Pollock98,Reference Payne, Prasad and Steiner103,Reference Sun, Liu and Wang115,Reference Uemura, Kuratsu and Hamada120 Of note, most reports are of small cohorts, single-center retrospective series, with only a few case series describing >20 patients, thus limiting the validity of any statistical analysis.Reference Penel, Amela and Decanter122,Reference Shinder, Jackson, Araujo, Prieto, Guadagnolo and Esmaeli123 A comprehensive search reveals a total of 17 studies now published reporting the utility of SRS for recurrent and residual HPC, summarized in Table 2.Reference Cohen-Inbar, Lee and Mousavi10,Reference Coffey, Cascino and Shaw14,Reference Galanis, Buckner and Scheithauer17,Reference Chang and Sakamoto24,Reference Kano, Niranjan and Kondziolka27,Reference Sheehan, Kondziolka and Flickinger28,Reference Olson, Yen and Schlesinger47,Reference Ecker, Marsh and Pollock98,Reference Payne, Prasad and Steiner103,Reference Kim, Kong and Seol104,Reference Kim, Kim and Chung106,Reference Sun, Liu and Wang115,Reference Iwai and Yamanaka124–Reference Tsugawa, Mori and Kobayashi127 The first of the reports is by Coffey et al.Reference Coffey, Cascino and Shaw14 describing a small cohort of 11 lesions in five patients, all receiving prior craniotomy (three also received prior EBRT with doses of 50–53 Gy). Prescribed margin doses reported ranged 12–18 Gy, and the mean follow-up period was 14.8 months. Eight of the nine treated tumors decreased in size significantly.Reference Coffey, Cascino and Shaw14 An overlapping cohort of 10 patients and 20 lesions was reported by Galanis et al.Reference Galanis, Buckner and Scheithauer17 (including the 5 patients reported by Coffey et al.Reference Coffey, Cascino and Shaw14), treated with salvage SRS (12–18 Gy). In this cohort seven patients failed prior EBRT 30.6–64 Gy, and all treated lesions showed volume control after SRS (14 decreased in size, 4 disappeared, 2 stable in size).Reference Galanis, Buckner and Scheithauer17 Tian et al.Reference Tian, Hao and Hou128 noted that when HPC recurrence is diagnosed before age 35 years, this serves as a significant negative prognostic predictor of an earlier second relapse and shorter OS.Reference Tian, Hao and Hou128 Payne et al.Reference Payne, Prasad and Steiner103 reported a cohort of 12 patients (15 HPC lesions) treated with a mean prescription dose of 14 Gy (2.8–25 Gy). During a mean clinical follow-up period of 24.8 months, nine lesions decreased in size and three lesions remained stable. Of note, late progression was noted after 22 months.Reference Payne, Prasad and Steiner103
Table 2: Cranial hemangiopericytoma SRS series
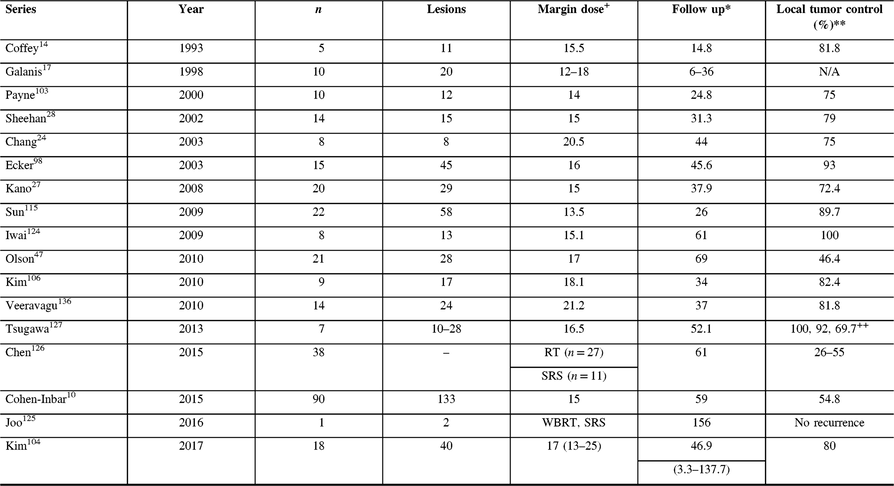
WBRT = whole brain radiotherapy; RT = radiotherapy; SRS = stereotactic radiosurgery.
+ Gy.
* Median, months.
** At the last follow-up.
++ At 1, 3, and 5 years, respectively.
Sheehan et al.Reference Sheehan, Kondziolka and Flickinger28 reported a cohort of 14 patients harboring 15 lesions treated with SRS. This patient cohort underwent a total of 27 prior craniotomies and 7 EBRT courses (total doses of 30–61 Gy, mean 47.3 Gy). The salvage SRS prescription dose was 11–20 Gy, and the follow-up period was 5–76 months (average 31.3). Tumor regression (volume reduction) was shown in 12/15 lesions. The 5-year local tumor control and survival rates were 76% and 100%, respectively, and DNM was reported in 29%. A mean prescription (margin) dose >15 Gy was shown to result in >50% reduction of tumor volume in 80% of patients.Reference Sheehan, Kondziolka and Flickinger28 Similar conclusions can be found in a report by Kano et al.Reference Kano, Niranjan and Kondziolka27 of a cohort of 20 patients (29 lesions) treated with SRS. The authors reported significantly better PFS (p = 0.0023) when patients were treated with a margin dose >14 Gy, with a 5-year PFS of 75.4% versus 56.3%, respectively (p = 0.0023).Reference Kano, Niranjan and Kondziolka27 DNM or ENM developed in 79.2 (range 12.2–158.3) months on average after the initial diagnosis. Local tumor control rates (volume reduction or stability) were 72.4% (n = 21), 20% (n = 4) died of DNM, and 5% (n = 1) died of ENM (liver and lung). Complete resolution was noted in 17.2% (n = 5) of the WHO-II HPCs.Reference Kano, Niranjan and Kondziolka27 Chang and SakamotoReference Chang and Sakamoto24 reported the use of a higher prescription dose (16–24 Gy, mean 20.5 Gy) in a cohort of eight HPC patients, achieving a 75% (n = 6) tumor reduction rates during a mean follow-up of 44 months (range 8–77).Reference Chang and Sakamoto24 Ecker et al.Reference Ecker, Marsh and Pollock98 reported a cohort of 38 patients (n = 22 grade-II, n = 16 grade-III HPCs), all treated with EBRT with or without SRS.Reference Stessin, Sison and Nieto50 A grade-III HPC histology was shown to result in recurrence significantly earlier (3.3 vs. 10 years, p = 0.004). The authors concluded that harnessing SRS for the treatment of recurrent disease contributed to a better OS.Reference Ecker, Marsh and Pollock98
Melone et al.Reference Melone, D’Elia and Santoro7 reported a series of 36 HPCs. The actuarial 5- and 10-year recurrence rates were 50% and 72%, respectively. Adjuvant ionizing radiation (all modalities, mean prescription dose 16 Gy) was shown to significantly decrease the rates of recurrence (p = 0.04), not improving OS (p = 0.2).Reference Melone, D’Elia and Santoro7 Kim et al.Reference Kim, Kong and Seol104 recently published a retrospective analysis of 18 patients (WHO-II = 8, WHO-III = 10) and 40 lesions (WHO-II = 13, WHO-III = 27) treated with SRS. The median OS was 134.7 months, and the actuarial survival rates at 1, 5, and 10 years were 85.6%, 85.6%, and 37.4%, respectively. Local tumor control was 80% (n = 32), and the average local recurrence-free interval (per lesion) for WHO-II and WHO-III were 86.1 and 40.5 months, respectively (p = 0.010). ENM developed in seven (38.9%) patients with WHO-III HPCs.Reference Kim, Kong and Seol104
Jeon et al.Reference Jeon, Park and Kim129 recently reported on the efficacy of adjuvant RT in a cohort of 49 patients with intracranial HPC. Of the cohort, 31 patients received adjuvant RT after surgery, 26 with EBRT and 5 with SRS (Gamma Knife). The median follow-up period was 50 months (range 3–216). The authors concluded that local tumor control was better with GTR followed by RT than GTR alone (p = 0.056), with no difference in OS. Local tumor control and OS after STR + RT were equivalent to those after GTR alone. Tumor volume >40 cm3 was associated with poor PFS (p = 0.024).Reference Jeon, Park and Kim129
In 2015, we reported the largest HPC cohort to date, a collaborative effort through the International Gamma Knife Research Foundation (IGKRF), done in an attempt to study outcomes of SRS for HPCs.Reference Cohen-Inbar, Lee and Mousavi10 Eight centers pooled patients together to form a cohort of 90 patients and 133 discrete lesions (78.9% n = 71 WHO-II, 21.1% n = 19 WHO-III). Prior treatment modalities included embolization (n = 8), chemotherapy (n = 2), and EBRT (n = 34). The median tumor volume was 4.9 cm3 (0.2–42.4 cm3), the median prescription dose was 15 Gy (2.8–24 Gy), and the median follow-up period was 59 months (range 6–190). Local tumor control was noted in 55% of tumors and 62.2% of patients. DNM was noted in 27.8% of patients, and ENM was noted in 24.4%. The actuarial OS at 2, 4, 6, 8, and 10 years was 91.5%, 82.1%, 73.9%, 56.7%, and 53.7% respectively. Local PFS at 2, 4, 6, 8, and 10 years was 81.7%, 66.3%, 54.5%, 37.2%, and 25.5%, respectively, after initial SRS (Figure 1).Reference Cohen-Inbar, Lee and Mousavi10
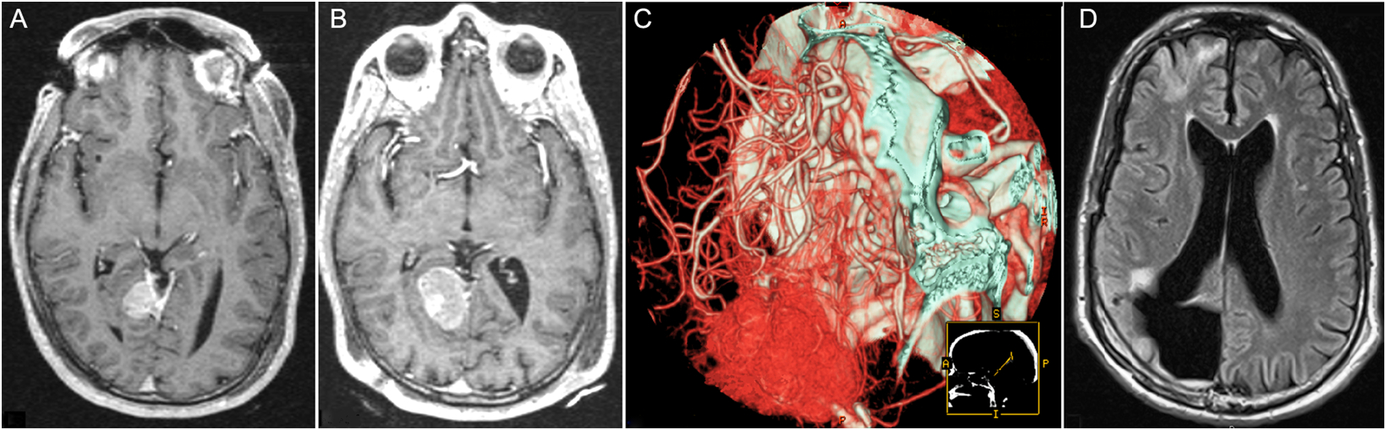
Figure 1: Sample Patient, Hemangiopericytoma. (A) Axial T1WI with gadolinium taken on the day of stereotactic radiosurgery (SRS) for a right tentorial hemangiopericytoma, s/p 1 surgical resection, 4/2002. (B) Axial T1WI with gadolinium taken on the day of repeat SRS for the same lesion, 12/2005. (C) CT-angio reconstruction pre-embolization and second surgical resection of the same lesion due to progression, 6/2009. (D) Follow-up images, 20 months after the second surgical resection, in T1WI-FLARE, showing final local control, 02/2011. Adapted from Cohen-Inbar et al.Reference Cohen-Inbar, Lee and Mousavi10
There are no cohort reports on outcome after SRS for spinal HPC, rather a few isolated case reports, all depicted and described in Table 1.Reference Schirger, Uihlein and Parker52–Reference Sweid, Noureldine and Nasser96 Our understanding on the role of SRS in treating spinal HPC is based on the cranial HPC’s series reports. The patient-friendly design and supporting science and data of SRS makes this a valuable tool in our armamentarium, and future studies are required to directly prove its role in the management of spinal HPC.
Distant Neural Metastasis
The incidence of DNM varies widely in reports, ranging 14–63.6%.Reference Cohen-Inbar, Lee and Mousavi10,Reference Coffey, Cascino and Shaw14,Reference Sheehan, Kondziolka and Flickinger28,Reference Olson, Yen and Schlesinger47,Reference Payne, Prasad and Steiner103,Reference Kim, Kim and Chung106,Reference Sun, Liu and Wang115 We reported a 27.8% (n = 25) incidence of DNM,Reference Cohen-Inbar, Lee and Mousavi10 whereas Kim et al. reported a 44.4% (19 lesions in eight patients).Reference Kim, Kong and Seol104 The WHO pathological grade strongly affects the risk of DNM (37.5% in grade-II and 50% in grade-III). One important clinical implication stemming from the different reports is that new DNM lesions (i.e., out-of-field recurrence) can be effectively treated with additional SRS sessions. Since the cumulative long-term risk of DNM development is high, a vigilant radiographic and clinical follow-up visit schedules are mandated.Reference Kim, Kong and Seol104
Extra-Neural Metastasis
HPCs are notorious for sending ENM, unlike most other primary brain neoplasms. Common ENM HPC sites are the liver, lung, bones, abdominal cavity, kidney, and pancreas.Reference Mena, Ribas and Pezeshkpour4 The incidence of ENM ranges 11.1–25.0% in different reports with a lag time of 8–16 years after initial diagnosis.Reference Kim, Kong and Seol104,Reference Koyama, Harada and Nakao130 We reported an ENM incidence of 24.4% (n = 22), located in the liver, lung, kidney, bone, bowel, and external auditory canal. In our series, the median time to ENM was 21.5 months (range 3–108 months).Reference Cohen-Inbar, Lee and Mousavi10 Kim et al.Reference Kim, Kong and Seol104 reported a series with impressive follow-up durations, which may explain the unusually high reported ENM rates of 38.9%. One clear observation is that ENM serves as a late event in HPC disease course, related to histopathological grading (more prevalent in Grade-III HPC’s),Reference Kim, Kong and Seol104 serving as a negative prognosticator and an harbinger of treatment failure. ENM is an accepted negative indicator of diminished OS, with a mean 24 months after ENM identification.Reference Chang and Sakamoto24 Therefore, a vigilant long-term systemic surveillance for ENM is pivotal, in addition to local and DNM surveilance.
Prognostic Factors Associated with Tumor Control and Overall Survival
When reviewing parameters that influence tumor local control rates’ OS, many such parameters suggested in shorter follow-up series do not maintain their statistical significance in longer follow-up series. We reported two major tumor- and treatment-related parameters that were shown to be significant and prognostic: prescription margin dose >16 Gy (p = 0.037, 95% CI 0.224–0.956 for univariate analysis; p = 0.039, 95% CI 0.194–0.968 for multivariate analysis), and tumor grade (p = 0.006; 95% CI 0.1382–6.616 for univariate analysis; p = 0.011, 95% CI 1.047–5.045 for multivariate analysis). Target SRS volume (i.e., tumor volume) was shown to be prognostic only in univariate analysis (p = 0.048), but bear in mind that this report focused on single-session SRS session, thus inherently featuring volume constraints (typically less than 10 ml for single-session SRS, median volume 4.9 cm3 in our series).Reference Cohen-Inbar, Lee and Mousavi10 In our multicenter series,Reference Cohen-Inbar, Lee and Mousavi10 OS was influenced solely by ENM presence (p = 0.029, 95% CI 1.103–6.323). WHO grade was reported by others to influence OS.Reference Kim, Kong and Seol104
Treatment of Local Tumor Recurrence
HPCs’ aggressive and relentless nature portrays a poor prognosis, one in which recurrence is the rule, not the exception. Thus, most patients will require more than one treatment modality.Reference Cohen-Inbar, Lee and Mousavi10 Patients in good physical condition and low operative risk, harboring a potentially resectable recurrent disease, should be considered for repeat microsurgical resection.Reference Payne, Prasad and Steiner103 Such surgical challenges have added complications and risk of reoperation to a vascular lesion, on top of common surgical risks and complications. Wang et al. recently reportedReference Wang, Zhang and Zhang131 a cohort of 57 patients with recurrent HPC (grades II–III), treated during 2008–2016. At the first recurrence, 30 patients (52.6%) underwent surgery, 25patients (43.9%) declined surgery, and 2 patients (3.5%) received Gamma Knife treatment. The 1-, 3-, and 5-year actuarial rates of second PFS was 73.3%, 46.7%, and 24.9%, respectively, for HPC grade-II and 66.7%, 66.7%, and 0%, for HPC grade-III, respectively. The actuarial 1-, 3-, and 5-year OS after the first recurrence were 87.4%, 69.2%, and 39.5% for HPC grade-II and 85.2%, 45.9%, and 24.5% for HPC grade-III, respectively. Each 1-month increase in the time interval from first surgery to first recurrence (first recurrence-free survival) (HR, 0.972; 95% CI, 0.952–0.993; p = 0.010) was noted to be strongly associated with better OS. Patients who received surgery with or without radiation at their first recurrence survived longer than patients who did not (53.0 vs. 35.7 months; p = 0.028).Reference Wang, Zhang and Zhang131 Thus, the decision to recommend reoperation in these should carefully balance tumor-related features, patient-related features, patient and surgeon’s inclinations, and always be preceded by an honest and complete patient consult. Alternatively, the hyper-vascular nature of HPCs suggests that immunotherapeutic antiangiogenic strategies (e.g., bevacizumab) might be another feasible option, although results are scarce and quite disappointing in recurrent HPC treatment as of now.Reference Ecker, Marsh and Pollock98 Still, this concept is attractive since no SRS treatment can prevent DNM.Reference Kano, Niranjan and Kondziolka27,Reference Sheehan, Kondziolka and Flickinger28,Reference Payne, Prasad and Steiner103,Reference Kim, Kim and Chung106
Repeat SRS has been utilized in the treatment of recurrent HPC, yet the optimal dosing is still a matter of debate, balancing the higher risk of adverse radiation effects to the desire to achieve tumor volume control (both increase with increasing dose).Reference Sun, Liu and Wang115 The mean prescription doses reported for repeat SRS range 13.5–17 Gy.Reference Coffey, Cascino and Shaw14,Reference Galanis, Buckner and Scheithauer17,Reference Chang and Sakamoto24,Reference Kano, Niranjan and Kondziolka27,Reference Sheehan, Kondziolka and Flickinger28,Reference Olson, Yen and Schlesinger47,Reference Ecker, Marsh and Pollock98,Reference Payne, Prasad and Steiner103,Reference Sun, Liu and Wang115,Reference Penel, Amela and Decanter122,Reference Alén, Lobato and Gómez132 Olson et al.Reference Olson, Yen and Schlesinger47 reported PFS rates of 90%, 60.3%, and 28.7% at 1, 3, and 5 years after initial SRS, respectively. These rates improved to 95%, 71.5%, and 71.5% at 1, 3, and 5 years after multiple SRS treatments.Reference Olson, Yen and Schlesinger47 We reported a cohort of 32 patients receiving 48 repeat SRS procedures for 76 lesions, of which 17 lesions were true recurrences (in-field), whereas 59 were DNM.Reference Cohen-Inbar, Lee and Mousavi10 With a median prescription dose of 14 Gy (range 12–16 Gy), the actuarial PFS at 2, 4, 6, and 8 years was 89%, 77%, 64%, and 54% after a second SRS treatment, respectively.Reference Cohen-Inbar, Lee and Mousavi10
Summary
Treating patients harboring cranial HPCs remains a partially answered challenge, owing to these lesions’ aggressive behavior. Resection (GTR when feasible and safe) remains the initial treatment option.Reference Trifiletti, Mehta and Grover133 For spinal HPCs, compartment location (ED/ID, IM/EM) coupled with histological grade is a crucial tumor-related parameter. Patient neurological functioning and overall health is another such parameter. Patients receiving surgery (GTR/STR) and RT (different modalities) show improved OS and PFS as compared to surgery alone or biopsy. We practice routine SRS or SBRT to the surgical bed even after GTR, as is a common practice in other malignant lesions (brain metastases, etc.). In many cases, patient-related comorbidities and tumor parameters (adjacent structures, skull-base location, etc.) preclude a GTR. EBRT or SRS does not impact OS in HPC patients. Their benefits are clearly limited to improved local tumor volume control and neurologic function, not affecting DNM or ENM development. In this scope SRS/ EBRT proves effective and safe and is thus supported by these authors, for both grade-II and grade-III HPCs.Reference Chang and Sakamoto24,Reference Sheehan, Kondziolka and Flickinger28,Reference Trifiletti, Mehta and Grover133 Kim et al.Reference Kim, Kim and Kong134 recently reported a retrospective study of outcome in SFT-HPC in a cohort of 10 SFTs, 33 HPC-grade II, and 4 HPC grade-III. Mean and median follow-ups were 114.6 and 94.7 months, respectively (range 7.1–366.7). GTR was shown to significantly positively affect PFS and OS (p = 0.012), and SRS/EBRT versus none led to significantly longer PFS (p = 0.018). SRS provides acceptable rates of local tumor volume control coupled with treatment safety and a patient-friendly apparatus and procedure. Single-session SRS is most effective for lesions measuring <2 cm their largest diameter or 10–12 cm3,Reference Cohen-Inbar, Lee and Mousavi10,Reference Schirmer and Heilman30 prescribing a margin dose of at least 15 Gy.Reference Someya, Sakata and Oouchi135 SRS leads to tumor volume control and neurological stability in most patients.Reference Cohen-Inbar, Lee and Mousavi10 For larger lesions, one may consider hypofractionated SRS or conventional EBRT. The management of recurrent intracranial HPC’s disease management must be tailored to the size and location of a specific lesion and to the overall systemic disease burden.Reference Schirmer and Heilman30 Repeat GTR can be attempted yet in most cases, and a planned STR followed by interval planned SRS is safer and more effective (AHS approach).Reference Cohen-Inbar and Sviri107 Smaller recurrent HPC lesions can be adequately controlled with repeat or upfront SRS alone.Reference Cohen-Inbar, Lee and Mousavi10,Reference Schirmer and Heilman30 EBRT can serve a palliative role in widespread disease.
A key component to patient care lies in vigilant clinical and radiographic follow-up visit schedule. Close follow-up imaging schedules and an alert team unequivocally lead to early detection of tumor recurrence, ENM or DNM development. Early detection benefits patients allowing to target smaller tumor volumes, making adjuvant SRS an even more attractive option. Follow-up intervals of 6 months seem prudent and sufficient. Since local recurrences, DNM, and ENM may develop many years after the initial diagnosis, follow-up should proceed indefinitely.
Conflict of Interest
The author has no conflicts of interest to declare.





