Part I: Historical
The idea of performing cerebral angiography, the precursor to interventional neuroradiology (INR), began with Egas Moniz in 1927. In the finest traditions of Portuguese exploration, he discovered a pathway for cerebrovascular navigation when he inadvertently opacified the human cerebral circulation using a direct carotid injection of strontium bromide. By 1930, Brooks proposed treating a traumatic carotid cavernous fistula (CCF) by direct injection of muscle into the internal carotid artery. In 1931, the Lancet proclaimed: “not only can cerebral angiography diagnose cerebral aneurysms, but its possibilities as an avenue for therapeutics should not be lost sight of in the future.” In a deceptively simple 6-page masterpiece from 1953 Reference Seldinger1 , Sven Seldinger described the transfemoral technique that still bears his name. This allowed unlimited opportunities for catheter-based intervention. The first documented cerebrovascular embolization came in 1960, when Baltimore neurosurgeon Alfred Luessenhop injected silicone beads directly into the internal carotid artery, hoping to thrombose a brain arteriovenous malformation (bAVM) Reference Luessenhop and Spence2 . By 1964, he helped develop a glass chamber that could propel a floppy, flow-directed silastic catheter into cerebral vessels. Arguably, the “father” of INR is Fedor Andreevich Serbinenko (Figure 1). Born in the USSR in 1928, he was trained as a machinist, then as a neurosurgeon at the Burdenko Institute in Moscow. He became proficient at direct cerebral angiography and earned a doctorate in CCFs in 1957. Surrounded by festive helium-filled balloons at the May Day parade in 1959, he wondered if a tiny balloon could be navigated into the cerebral circulation to occlude fistulae and aneurysms. In 1964, he navigated a silicone balloon into the cerebral circulation, and in 1969, he successfully treated a CCF. By 1973, he had performed over 160 balloon procedures, including a 2-balloon approach for wide-necked aneurysms and vascular dilatation for vasospasm. His results were published in 1974 Reference Serbinenko3 and the world took notice. Gerard Debrun, a French neuroradiologist, began working on his own detachable latex balloon system and visited Moscow in 1975. Charles G. Drake, then one of the world’s leading aneurysm and AVM surgeons, encouraged Debrun to join his team in London, Ontario. Simultaneously, centers for this new sub-specialty of INR sprang up at multiple sites in North America and Europe.

Figure 1: Fedor Andreevich Serbinenko, MD, PhD. 1928–2002.
The adhesive polymer iso-butyl-cyano-acrylate (IBCA) was developed in the 1960s as a possible alternative to sutures but was adapted in the 1970s into a liquid embolic agent for AVMs, delivered by an innovative, “calibrated-leak” balloon system perfected by Charles Kerber Reference Kerber4 . In 1986, the first flexible microcatheters and microguidewires were introduced, allowing precise delivery of embolic materials like coils and particles. There were many seminal publications in the 1980s describing balloon occlusion of giant aneurysms and fistulae, AVM obliteration with IBCA, and intra-aneurysmal balloon placement. In the mid-1980s, the Italian neurosurgeon Guido Guglielmi, working with Fernando Vinuela in Los Angeles, developed the tiny platinum microcoils that would revolutionize cerebral aneurysm treatment (Figure 2). Following the publication of their work in 1991 Reference Guglielmi, Vinuela and Dion5 , INR would soon become indispensable for anyone treating cerebrovascular disorders.

Figure 2: (A) Fernando Vinuela, Guido Guglielmi, Gary Duckwiler, University of California, Los Angeles, 1992. (B) Design sketch for the first detachable coil. (C) Detachable coil prototype. (D) The Guglielmi Detachable Coil (GDC). (E) The first GDCs for cerebral aneurysms. Courtesy of Dr. Fernando Vinuela.
Part II: Cerebral Aneurysms
The concept of mechanical or electrical thrombosis of aneurysms was proposed for aneurysms in the peripheral circulation in the 1800s and by Walter Dandy for cerebral aneurysms in the 1920s. Gallagher used horsehair in 1964, and Mullan used copper beryllium and platinum wire in 1965. Silicone and latex balloons were detached inside aneurysms in the 1970s. It was not until the advent of detachable platinum coils in 1991 that a reliable and effective endovascular option became available. Initially planned to induce electrothrombosis of the aneurysm lumen, it was soon realized that thrombosis was achieved by mechanical obliteration with thrombus formation and that the main function of the electrical current was for coil detachment (Figure 3). As the technique became popular, it was imperative to gather evidence of its efficacy.

Figure 3: Coiled cerebral aneurysm.
The landmark International Subarachnoid Aneurysm Trial (ISAT) first provided this in 2002 Reference Molyneux, Kerr and Yu6 . ISAT was a multicenter, randomized controlled trial (RCT) of clipping (1070 pts.) vs. coiling (1073 pts.) in patients with ruptured, anterior circulation aneurysms. Aneurysms had to be suitable for either clipping or coiling, resulting in 22% of screened lesions being entered. The study showed an absolute risk reduction of 6.9% and 24% relative risk reduction in the clinical outcome at 1 year for the coiled cohort. Medium-term results showed an increased need for re-treatment in the coil group with no adverse effect on clinical outcome. Long-term follow-up between 10 and 18.5 years showed that the proportion of patients with a good Modified Rankin Score (mRS) score (0–2) did not differ between the clip and coil groups. The probability of being alive with a good outcome compared with death or dependency was significantly better for the coiled group. The most recent conclusions of the study are that the durability and clinical benefit of aneurysm coiling noted at 1 year are maintained at 10 years. In both groups, the risk of death and rebleeding is very small and similar to the risk of recurrent hemorrhage from another aneurysm Reference Molyneux, Birks, Clarke, Sneade and Kerr7 .
Many studies and case series were published after ISAT. Non-randomized studies such as ATENA in 2008 Reference Pierot, Spelle and Vitry8 confirmed the safety and efficacy of coiling in 1100 unruptured aneurysms, with a 95% success rate, morbidity, and mortality at 1 month of 1.4% and 1.7%. CLARITY in 2010 Reference Pierot, Cognard, Andonnat and Ricolfi9 showed that coiling was effective in 782 ruptured aneurysms, but that thromboembolic complications were higher (28%) with larger aneurysms (>1 cm diameter) and that there was a significant rupture risk for middle cerebral artery (MCA) bifurcation aneurysms (8.5%). Cerecyte Reference Molyneux, Clarke and Mehta10 and MAPS Reference McDougall, Johnston and Barnwell11 showed no significant advantage of coils coated with bioactive substances over bare platinum coils, and HELPS Reference White, Lewis, Nahser, Sellar, Goddard and Gholkar12 showed that hydrogel coated coils may reduce recurrence rates.
The Barrow Ruptured Aneurysm Trial (BRAT) from 2012 Reference McDougall, Spetzler and Zabramski13 confirmed the results of ISAT, with better outcomes in the coiled group at 1 year. The 10-year follow-up of 362 patients showed that there was no significant difference in poor outcome (mRS>2) between clip and coil groups. Retreatment was necessary in 20% of the coiled and 0.8% of the clipped patients. There was complete aneurysm obliteration in 93% of the clipped and 22% of the coiled aneurysms. There were no bleeds in those who needed recoiling, but there was a 38% crossover rate from coiling to clipping. There were two fatal rebleeds, both in the coiled group Reference Spetzler, McDougall and Zabramski14 .
Technology has advanced dramatically since the patients from ISAT and BRAT were enrolled. Balloon and stent-assisted coiling (Figures 4 and 5), flow-diverting stents, and complex endoluminal constructs, the most popular being the Woven EndoBridge (WEB) device, are now part of the endovascular armamentarium.

Figure 4: Balloon-assisted coiling.

Figure 5: (A) Stent-assisted coiling. Right vertebral artery DSA, AP view. Basilar bifurcation aneurysm. (B) Right vertebral artery DSA, AP view. Post Y-stent-assisted coiling. (C) Unsubtracted AP view. Note radiopaque stent tips (arrowheads).
The first flow-diverting stent for treatment of intracranial aneurysms was performed in 2007 in Leiden, Holland. The Pipeline Embolization Device (PED) was approved for use in Europe in 2008 and in the US in 2011 (Figure 6). There are at least nine other similar devices in use or in development. None have been approved for use in the posterior fossa, in acute subarachnoid hemorrhage, or in the internal carotid artery beyond the superior hypophyseal segment. The PED is the most investigated device, and two studies in patients with unruptured, wide-necked aneurysms showed occlusion rates of 75%–95% at 6 months–5 years with low morbidity and mortality (<3%). Off-label usage, however, is more dangerous, with complication rates in ruptured aneurysms of 18% in the anterior circulation and 27% in the posterior circulation. Complication rates are higher with the use of three or more flow diverters, and for treatment of fusiform aneurysms, particularly in the posterior fossa Reference Dmytriw, Phan, Moore, Pereira, Krings and Thomas15 .

Figure 6: (A) Left internal carotid DSA, lateral view. Giant cavernous ICA aneurysm. (B) CT angiogram, sagittal view. PED in place. (C) Left ICA DSA, lateral view at 3 months. Complete thrombosis of aneurysm.
The WEB is a self-expanding, electrothermically detachable, and nitinol braided endoluminal device designed for wide-necked bifurcation aneurysms (Figures 7 and 8). A review of 169 aneurysms treated at three French centers, 89% of which were unruptured, showed that the device was successfully deployed in 96.4% of cases Reference Pierot, Moret and Barreau16 . Mid-term anatomic results were complete occlusion in 52.9%, neck remnant in 26.1%, and aneurysm remnant in 20.9%. The authors considered that the overall adequacy of the treatment was 79.1%. There was no rebleeding in any case and 6.9% needed retreatment. Immediate thromboembolic events were seen in 14.4%, but only 3% had permanent deficits. At 1 year, all disease and procedure-related mortality was 1.4% and morbidity was 1.3%. The authors believe that their results in this challenging group of patients compare favorably with surgical series of unruptured aneurysm treatment and The International Study of Unruptured Intracranial Aneurysms, a registry followed over time Reference Wiebers, Whisnant, Huston, Meissner and Brown17 .

Figure 7: The WEB device.

Figure 8: (A) Right vertebral artery DSA, AP view. WEB device deployed in basilar bifurcation aneurysm. (B) Contrast-enhanced MR angiography pre-op. (C) Contrast-enhanced MR angiography three months post-op showing complete thrombosis of the aneurysm.
The best management of unruptured intracranial aneurysms (UIAs), which are present in 1%–5% of the population, is largely unknown. There is no RCT evidence comparing active vs. conservative management or surgical vs. endovascular therapy (EVT). The PHASES and UIATS scores, developed from the available literature, are commonly used for best management, but they are still imperfect. There is one ongoing RCT of surgery vs. coiling (Canadian Unruptured Endovascular versus Surgery Trial [CURES]), but slow recruitment has delayed formulation of any conclusions Reference Darsaut, Findlay and Magro18 .
At many centers in the world, 70%–90% of cerebral aneurysms are now treated by EVT Reference Amenta, Nerva and Dumont19 . Despite the inexorable trend toward more expensive endovascular options Reference Wang, Campos, Colby, Coon and Lin20 , the debate between clipping and coiling remains lively Reference Cockroft21 . In the post-COVID era, collection and evaluation of robust data regarding anatomic occlusion rates, morbidity and mortality, length of stay, and total costs will be imperative. In many centers, a straightforward clipping by an experienced cerebrovascular surgeon may be preferable to a complex endovascular procedure. This may be limited by fewer direct surgical cases in residency training, leading to fewer experienced operators in the future. There will likely always be a need for a multidisciplinary, collegial, and collaborative approach to cerebral aneurysm treatment.
Part III: Acute Ischemic Stroke
Perhaps nowhere else has EVT made a greater immediate impact than on the management of acute ischemic stroke (AIS). Just as the introduction of intravenous alteplase (rtPA) in 1996 revolutionized acute stroke treatment, the publication of five RCTs in 2015 completely changed how these patients are imaged and treated.
The PROACT II Reference Furlan, Higashida and Wechsler22 Trial, published in 1999, was the first study to show some promise for EVT in AIS. A total of 180 patients with acute MCA occlusion of less than 6 h duration were randomized to either intra-arterial (IA) injection of 9 mg of pro-urokinase (r-proUK) plus heparin or IA heparin alone, delivered through a microcatheter in the proximal MCA. The primary analysis showed that 40% of the r-proUK patients and 27% of the control heparin group had an mRS of 2 or less at 90 days. Secondary analyses, however, showed 25% and 27% mortality rates and 10% and 2% rates of intracranial hemorrhage (ICH) at 24 h for the r-proUK and control groups, respectively. The recanalization rates were 66% for the r-proUK group and 18% for the control group. Pro-urokinase, however, was not made widely available for clinical usage.
The US Food and Drug Administration (FDA) approved the MERCI mechanical thrombectomy device in 2004 and the Penumbra aspiration device in 2008. Multiple comparison studies with IV r-tPA were performed using these devices, including IMS-I, -II, and -III, Multi-MERCI, and a PENUMBRA study. They showed MCA recanalization rates of 46%–82%, mRS 0–2 at 90 days of 25%–46%, and symptomatic ICH rates of 6%–15%. The conclusions were that combined IA/IV therapy was not significantly better than IV alone. Stent retrievers such as Solitaire and Trevo became available in 2012, and initial comparison studies using these devices, including SWIFT, TREVO II, SYNTHESIS, DEFUSE 2, and MR Rescue, also showed no significant advantage of IA over IV therapy.
Everything changed in 2015. Five RCTs were published which conclusively demonstrated that mechanical thrombectomy could dramatically improve outcomes in AIS caused by intracranial large vessel occlusion Reference Goyal, Menon and van Zwam23 . The keys to success were the speed of treatment initiation, the use of stent retrievers in 85% of cases, and the lack of “cherry-picking,” or biased selection of study patients. Patient populations ranged from 70 to 502 and were treated within 6–12 h of symptom onset. MCA recanalization rates ranged from 72.4% to 88%, symptomatic hemorrhages between 0% and 7.7%, and mRS 0–2 at 90 days of 32%–72%. Mortality rates were between 9% and 21%. The number needed to treat (NNT) to reduce disability by at least one level on mRS for one patient was 2.6.
Suddenly, speed was of the essence for success. In ESCAPE Reference Goyal, Demchuk and Menon24 , the median time from symptom onset to 1st reperfusion was 4 h. The median time of computerized tomography (CT) to 1st reperfusion was 84 min, and median time from groin puncture to 1st reperfusion was 30 min. Neurointerventional teams were quickly reorganized to facilitate a huge increase in eligible AIS patients. Questions remaining included the best imaging protocol for appropriate patient selection (plain CT, multiphase CT angiography [CTA], CT perfusion, and diffusion-weighted magnetic resonance imaging [MRI]), how best to select patients from peripheral centers, whether to use intraprocedural conscious sedation or general anesthesia, and the number of neurointerventional centers needed per capita.
As experience with ever more efficient mechanical and aspiration thrombectomy devices accumulated, it became apparent that patients with even longer symptom onset to presentation times, and their imaging demonstrated good collaterals with a small ischemic core, could benefit from EVT. The DAWN trial Reference Nogueira, Jadhav and Haussen25 evaluated 206 pts. who presented up to 24 h after symptom onset and had a mismatch between the severity of clinical deficit and infarct volumes on CT perfusion or diffusion-weighted MRI (DWI). They were randomized into control and thrombectomy groups. The median interval between when the patient was last known to be well and reperfusion was 13.6 h. There was successful reperfusion in 84% of the EVT group. The utility-weighted mRS and functional independence at 90 days (49%) were significantly better in the EVT group. Rates of ICH and mortality did not differ between groups. These results were confirmed by the DEFUSE 3 Trial, in which CT and MRI perfusion was used to select patients for EVT. Patients with a small ischemic core and favorable core to penumbra ratio, last seen well 6–16 h who underwent EVT were shown to have better outcomes at 90 days than those who received standard medical therapy alone Reference Albers, Marks and Kemp26 .
EVT has now become a therapeutic option of choice for patients up to 24 h of last being seen well, with severe deficits, favorable imaging, and little disability at baseline (Figure 9). Patients in smaller communities without neurointerventional capabilities are being imaged locally and those with appropriate imaging are often transported long distances for possible EVT at tertiary care centers. There are continuing discussions regarding direct transfer or initiation of IV thrombolytics at the peripheral center prior to transfer (“drip and ship”). This has made the possibility of neuroprotection even more relevant. The ESCAPE-NA1 Trial Reference Hill, Goyal and Menon27 evaluated the eicosapeptide nerinetide in 1105 patients with AIS up to 12 h after the patient was last seen well who were selected to undergo EVT. The primary outcome was mRS of 0–2 at 90 days. The results showed no significant difference between the groups; however, an exploratory analysis showed that those who received alteplase and nerinetide had worse outcomes (mRS 0–2 in 49.8% at 90 days) than those who received nerinetide alone (mRS 59.3%). The significance of this finding is uncertain and requires confirmation. Nerinetide does not affect the activity of alteplase; however, an inhibitory effect of alteplase on nerinetide is plausible. Nerinetide may have the potential benefit of those AIS patients who are beyond the 4.5 h window for IV alteplase, but are still candidates for EVT.

Figure 9: (A) Right internal carotid artery DSA, AP view. Complete embolic occlusion of the right MCA (arrow). (B) Right internal carotid artery DSA, AP view, post-mechanical thrombectomy. The right MCA is now patent.
There are many unanswered questions. It is still unclear whether patients with low NIHSS and proximal occlusions should undergo EVT. Improving technology now allows access to distal branch occlusions, but evidence for efficacy of EVT in these cases remains anecdotal. Is multiphase CTA adequate for pre-EVT evaluation, or is CT perfusion still necessary, as specified in many provincial guidelines? Should expedited MRI with DWI be a part of initial imaging? Despite abundant anecdotal evidence, RCT evidence of EVT in the posterior circulation over standard medical therapy is still lacking, the most recent trial being underpowered with excessive crossovers Reference Liu, Dai and Ye28 .
Part IV: Carotid Angioplasty and Stenting
Dotter and Judkins first described percutaneous transluminal angioplasty for treating arterial stenosis in 1964 Reference Dotter and Judkins29 . Application of EVT to the carotid circulation lagged behind due to the unacceptable consequences of distal emboli to the brain. The first carotid angioplasty was performed by Mullan in 1980 Reference Mullan, Duda and Patronas30 . By 1984, many investigators began treating patients with CAS. It was an attractive option perceived by many to be less invasive than carotid endarterectomy (CEA) (Figure 10). There was no skin incision, no general anesthesia, no risk of cranial nerve injury, and a shorter hospitalization. One of the largest initial series in 1996 described results in 259 pts., 74% of whom were symptomatic. It showed that the procedure could be successfully performed in a high percentage of patients with low perioperative complication rates Reference Theron, Payelle, Coskun, Huet and Guimaraens31 . By 2001, a large US series Reference Roubin, New and Iyer32 reported a 98% success rate, 30 days stroke, and death rates of 8.2% for symptomatic and 6.3% for asymptomatic patients. A global registry from 2000 showed over 94% technical success rate, perioperative minor stroke of 2.7%, and a major stroke rate of 1.5% and mortality of 0.86% Reference Wholey, Wholey and Mathias33 .

Figure 10: (A) Right common carotid artery DSA, lateral view. Severe right internal carotid artery stenosis (arrow). (B) Right common carotid artery DSA, lateral view post-CAS.
The surgical treatment of atherosclerotic stenosis of the cervical internal carotid artery was definitively validated by NASCET in 1991 34 . This landmark study showed a robust 17% stroke reduction in 2 years. Stroke risk in patients undergoing CEA was compared to ASA control patients, with a 30-day perioperative stroke risk of 5.8%. Despite improvements in medical management and the rapid evolution of endovascular technology, CEA was and remains the gold standard to which all other therapies are measured. By 2000, the time was right for RCTs of CEA vs. CAS.
The first was CAVATAS 35 in 2001. In 504 pts., 97% of whom were symptomatic, there was an unacceptable 10% risk of stroke or death in both groups followed for 3 years. SAPPHIRE Reference Yadav, Wholey and Kuntz36 in 2004 reported results on a group of 334 patients, 71% of whom were asymptomatic, harboring severe carotid stenoses and deemed to be at high risk for CEA. The risk of any stroke, death, or MI was 20% in the CEA group and 12% in the CAS group. This trial was very influential in obtaining FDA approval for CAS in the USA. The EVA 3S trial from 2006 was terminated early due to the significant risk of any periprocedural stroke or death in the CAS group (9.6%) compared to CEA (3.9%). At 4 years, the risk of ipsilateral stroke was equivalent. The SPACE trial in 2008 reported similar risks of ipsilateral stroke and death in 1200 symptomatic pts. followed for 2 years.
The two most influential RCTs are ICSS and CREST, both published in 2010. ICSS Reference Bonati, Dobson and Featherstone37 reported on 1733 symptomatic patients and showed that the risk of any stroke, death, or MI at 120 days was 5.1% in the CEA group vs. 8.5% in the CAS group. There were twice as many strokes with CAS (7% vs. 3.3% with CEA), and the initial conclusion was that CEA should be the treatment of choice. At long-term follow-up to 10 years, CAS was as effective as CEA in preventing fatal or disabling stroke in symptomatic patients with severe carotid stenosis. CREST Reference Brott, Hobson and Howard38 reported on 2502 patients, 47% of whom were asymptomatic. The primary endpoints of any stroke, death, or MI were considered equal at 4 years. (7.2% for CAS, 6.8% for CEA). There were more strokes with CAS (4.1% vs. 2.5%) and more MIs with CEA. The conclusion of these studies essentially established equipoise between CAS and CEA, acknowledging that there is a higher risk of minor, non-disabling periprocedural, and possibly long-term stroke with CAS, particularly in older patients. This must be weighed against the higher risks of MI, cranial nerve palsy, and access site hematoma with CEA.
Since then, CAS has been an attractive option for carotid revascularization in symptomatic and asymptomatic diseases. It is performed by a variety of practitioners, including cardiologists (approximately 50% in 2016), vascular surgeons, interventional radiologists, neurologists, and neurosurgeons. There is a general belief that embolic protection devices (EPDs) are necessary to reduce stroke risk, although this is not a universal sentiment. There are no standard protocols regarding patient selection, choice of preoperative imaging, vascular access (transfemoral, transradial, or transcarotid), method of embolic protection (proximal, distal, and flow-reversal), type of stent (open or closed cell), need for pre-or post-stent angioplasty, perioperative blood pressure management, modality, and length of follow-up.
Currently, approximately 80%–90% of carotid interventions in the US are done on asymptomatic pts. Reference Lichtman, Jones and Leifheit39 . ACAS in 1995 and ACST in 2004 showed that intervention could reduce the stroke risk by approximately 1%/year, but the NNT to prevent one stroke was 83 and morbidity and mortality must be < 3% to show any benefit Reference Bogiatzi, Azarpazhooh and Spence40 . The best management of asymptomatic carotid stenosis is still controversial, considering that optimal medical therapy (OMT) has dramatically improved since the original trials of CEA and CAS, and may reduce the annual stroke risk to approximately 0.5% Reference Derdeyn41 . It is estimated that only 5%–15% of asymptomatic carotid stenosis patients may benefit from an intervention Reference Spence42,Reference Abbott, Brunser and Giannoukas43 . High-risk imaging features included stenosis progression, plaque volume increase and echolucency on carotid Doppler, microemboli on transcranial Doppler, intraplaque hemorrhage on MRI, and reduced cerebrovascular reserve on perfusion imaging Reference Paraskevsas, Veith and Spence44 . Not all of these factors, however, have been adequately evaluated in the context of current OMT.
A meta-analysis of five RCTs of CAS vs. CEA for asymptomatic disease Reference Moresoli, Habib, Reynier, Secrest, Eisenberg and Filion45 found that CAS might potentially increase the risk of periprocedural stroke and death and recommended CEA in selected patients as a safer and more effective therapy. There are currently two large RCTs of CAS vs. CEA in asymptomatic patients underway, ACST II and CREST II. ACST II intends to recruit 3600 patients and to report initial results in 2021. CREST II is actually two trials, one of CAS+OMT vs. OMT alone and CEA + OMT vs. OMT alone. A recent preliminary report on 221 patients from the CREST II Registry, 40% of whom were symptomatic, showed that periprocedural stroke and death rates were 2.8% in symptomatic patients and 1.4% in asymptomatics Reference Giri and Olin46 . These low complication rates were attributed to better OMT regimens, better blood pressure control, and better technical factors including judicious use of EPDs; smaller, more compliant balloons; selective omission of pre- or post-stent angioplasty; and use of closed cell stents.
A pooled analysis of the four largest RCTs of CAS vs. CEA, including 4775 pts. followed for up to 12.4 years, concluded that periprocedural risks were greater with CAS, but post-procedural risks were similar, indicating the durability of both procedures. CEA is safer in patients over 75 years old Reference Brott, Calvert and Howard47 . Contemporary guidelines in the US and Europe continue to recommend CEA as the treatment of choice for severe (70%–99%) symptomatic carotid stenosis in average surgical risk patients, with periprocedural risks of < 6% (Class I, Level A). In high-risk surgical patients (coexistent medical morbidity, unfavorable anatomy, radiation-induced stenosis, and post-CEA restenosis), CAS should be considered as a therapeutic option (Class IIa, Level B). In asymptomatic patients with average or high surgical risk and 60%–90% stenosis, CEA is recommended if there are one or more high-risk imaging features (described above) and periprocedural complication rates <3% (Class IIa, Level B). CAS may be considered in high surgical risk patients (Class IIb, Level B). For average surgical risk patients, CAS may be considered in highly selected patients, but effectiveness compared to OMT has not been established Reference White48,Reference Naylor49 .
Part V: Intracranial Atherosclerotic Disease
Intracranial atherosclerotic disease (ICAD) is estimated to cause 10%–15% of ischemic strokes. There were two reports of balloon angioplasty for basilar artery stenosis in 1980 and many reports of EVT for MCA stenosis in the 1990s using coronary balloons and stents Reference Al-Mubarak, Gomez, Vitek and Roubin50 . Evolution of this procedure was slow due to the unsuitability of coronary devices for the cerebral circulation and relatively high rates of distal emboli, plaque dissection, perforator obstruction, and vessel rupture. A meta-analysis from 2009 Reference Siddiq, Memom, Vazquez, Safdar and Qureshi51 comparing symptomatic ICAD patients treated with angioplasty alone (1027 pts.) to those treated with angioplasty and stenting (1291) showed that 30 days and 1-year stroke and death rates were higher in the angioplasty-alone group (8.9% vs. 8.1% and 19.7% vs. 14.2%). Technical success was higher with angioplasty and stenting (95% vs. 79.8%) and restenosis was lower (11.1% vs. 14.2%). The conclusion was that angioplasty and stenting are superior to angioplasty alone for EVT of ICAD (Figure 11).

Figure 11: (A) Right internal carotid artery DSA, AP view. Severe right MCA stenosis (arrow). (B). Unsubtracted AP view. Angioplasty balloon in place. (C) Right common carotid artery DSA, AP view. Post-angioplasty.
The publication of the SAMMPRIS trial in 2011 essentially extinguished the procedure as a therapeutic option for ICAD Reference Chimowitz, Lynn and Derdeyn52 . A total of 451 patients with symptomatic ICAD were randomly assigned to medical management or EVT with the dedicated Wingspan stent and Gateway balloons (Stryker Neurovascular, Kalamazoo, MI). The trial was stopped early due to unexpectedly high rates of stroke or death in the EVT group and lower than expected rates of stroke and death in the medical group at 30 days (14.7% vs. 5.8%) and estimated at 1 year (20.0% vs. 12.2%). These results were confirmed in the smaller VISSIT trial in 2015 Reference Zaidat, Fitzsimmons and Woodward53 . For all practical purposes, there was no official endovascular option for symptomatic ICAD.
The procedure has, however, refused to die. SAMMPRIS showed that even in the medical group, 12% of patients had a stroke or death at 1 year. A Chinese study from 2015 Reference Miao, Song and Liebeskind54 demonstrated the feasibility and safety of EVT in a cohort of 158 pts. who presented with symptoms related to hypoperfusion and poor collateral flow. The composite 30-day risk of stroke, MI, or death was 4.4%. There are currently several ongoing Chinese RCTs on EVT for ICAD Reference Abualhasan, Abd-Allah and Pero55 . The lead-in phase of the CASSISS study showed a 30-day stroke and death rate of 2% in 100 pts Reference Yu and Jiang56 .
Submaximal angioplasty has recently become a popular alternative to traditional full balloon angioplasty and stenting. Even a minor increase in vessel diameter can result in a major increase in blood flow. Slow (1 atm/min.), incomplete balloon expansion can address symptoms of hypoperfusion while theoretically avoiding the known complications caused by full or overexpansion. A meta-analysis from 2020 Reference Seyedsaadat, Yolcu and Neuhaus57 described results in 777 symptomatic patients with ICAD, 491 in the anterior circulation, and 288 in the posterior circulation. Mean follow-up was 2.6 years, overall technical success rate was 93%, and stenoses were reduced from 50% to 80% to less than 20%. Periprocedural complications were 24.6% and at 30 days, the stroke rate was 2%, and death rate was 1%. At 1 year, the stroke rate was 4% and mortality 2%. The largest Chinese series from 2019 showed a 97% success rate, periprocedural complications of 5.4%, and a 30-day to 1-year stroke rate of 2.3%. Explanations for better results in Chinese studies may include highly experienced operators; more varied technical options; younger age groups; less racial heterogeneity; fewer comorbidities like coronary artery disease and dyslipidemia; presentation with TIAs rather than strokes, mainly proximal M1 stenoses; and longer intervals to treatment, possibly allowing plaque stabilization.
Current guidelines do not support EVT for ICAD. A recent study in carefully selected ICAD patients Reference Alexander, Zauner and Chaloupka58 , using the same technology as SAMMPRIS, showed that periprocedural complication rates could be reduced to 2.6%. Despite careful patient selection, increasing operator experience, and technological evolution, another RCT with OMT is needed before EVT can become a recommended therapy for ICAD.
Part VI: Brain AVMs (bAVMs)
The first published neurointerventional case described the embolization of a bAVM by Luessenhop in 1960. The primary goal for the treatment of these challenging lesions is to prevent ICH, but the actual annual hemorrhage risks for both ruptured and unruptured lesions remain controversial. A large meta-analysis from 2014 Reference Kim, Al-Shahi, McCulloch, Stapf and Young59 reported an overall annual risk of 2.3% per year over 10 years, 4.8% per year for ruptured, and 1.3% per year for unruptured bAVMs. Most bAVMs are discovered as incidental findings in patients being investigated for other reasons, appearing in up to 0.05% of all brain MRI examinations. The goals of EVT have evolved with time and experience Reference Derdeyn, Zipfel and Albuquerque60 .
Early embolic agents included metallic and silicone spheres, polyvinyl alcohol particles (PVA), and pushable coils. These were flow directed and ultimately made no difference to AVM nidus size or hemorrhage risk. The introduction of the liquid embolic agents IBCA in the late 1970s, the less toxic n-butyl-cyanoacrylate (n-BCA, Histoacryl [B Braun, Hessen, Germany], Trufill [Johnson and Johnson Medical, Raynham, MA]) in the 1980s, and ethyl vinyl alcohol copolymer (EVOH, Onyx [ev3 Endovascular, Irvine, CA]) in the late 1990s allowed more accurate and comprehensive nidal obliteration, initially via flow-directed silastic catheters and then using over-the-wire microcatheters. It soon became apparent that complete endovascular nidal obliteration was difficult to achieve and relatively uncommon. Complete cure rates were generally reported as approximately 20%, with higher rates reported in several series, mainly in small, non-complex lesions, which were also amenable to surgical resection. Complications of embolization included thromboembolic stroke from catheterizations, non-target embolization, vessel wall injury and perforation, bAVM rupture due to abrupt hemodynamic alteration, and venous outlet occlusion. A recent systematic review of embolization with intent to cure Reference Wu, Ahmadieh and McDougall61 showed that complete obliteration could be obtained in 45.8% of 597 patients, 34% of whom had Spetzler-Martin Grade III lesions. The overall clinical complication rate was 24.1%; most commonly due to hemorrhage (9.7%) with procedure-related mortality of 1.5%. The conclusions were that complications were unacceptably high when complete cure was attempted and that this should not be the primary goal of embolization.
The goals of embolization changed, evolving into an adjunct therapy prior to radiation or surgical excision (Figures 12 and 13). The ultimate objective is to reduce intraoperative bleeding or to decrease nidal diameter <3 cm, arguably more favorable for radiosurgery Reference Andrade-Souza, Ramani and Scora62,Reference Marks, Marcellus and Santarelli63 . Secondary goals are to eliminate high-risk features such as associated symptomatic aneurysms and to reduce the effects of arteriovenous shunting, venous hypertension, and vascular steals. Hard evidence for efficacy of these interventions is lacking. Multimodality therapies for large bAVMs, using combinations of embolization, surgery, and radiotherapy, report obliteration rates of 38%–83% and permanent neurological morbidity rates of 4%–14%. Staged intervention, dividing large bAVMs into sections treated at 2–9 months intervals, has reported obliteration rates of 33%–74% and complication rates of 3%–13% Reference Kim, Al-Shahi, McCulloch, Stapf and Young59 .

Figure 12: (A) Right internal carotid artery DSA, AP view. Large right frontal AVM. (B) Unsubtracted AP view. Post-Onyx embolization. (C) Right internal carotid artery DSA, AP view. Post-Onyx embolization.
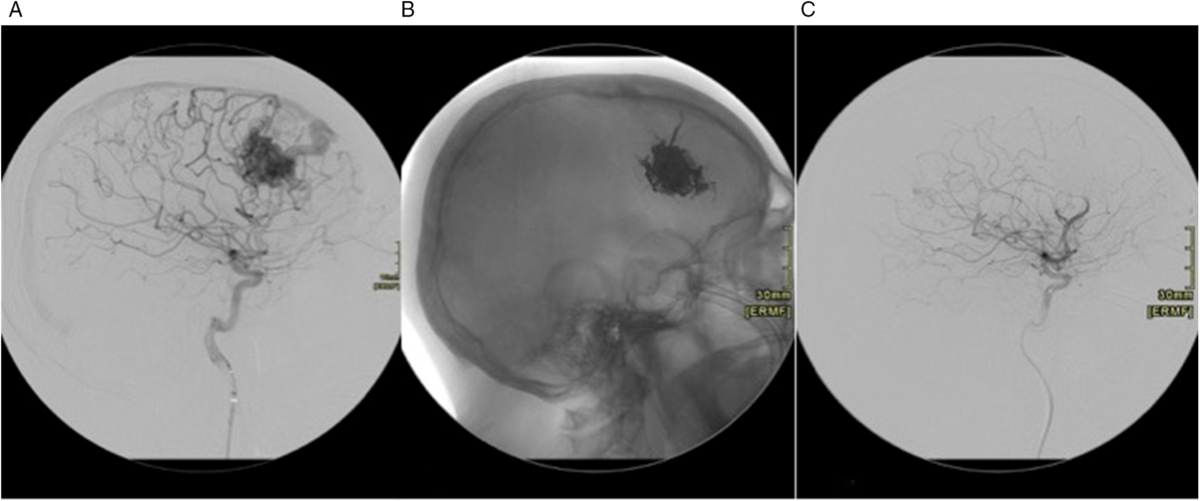
Figure 13: (A) Right ICA DSA, lateral view. Right frontal AVM. (B) Unsubtracted lateral view showing Onyx cast post-embolization. (C) Right ICA DSA, lateral view post embolization shows no filling of the AVM.
The approach to unruptured bAVMs has always been controversial, mainly due to the low annual risk of hemorrhage and the relatively high risk of complications from any type of therapy. The publication of ARUBA Reference Mohr, Parides and Stapf64 in 2014 was a landmark development. This RCT of medical treatment vs. medical treatment plus intervention for unruptured bAVMs was stopped early after recruitment of only 226 followed for a mean of 33 months. There was an unacceptably high risk of stroke and death in the interventional group (30.7% vs. 10.1%). An observational phase is continuing. A nonrandomized study from the same year showed similar findings Reference Al-Shahi, White and Counsell65 .
There have been many criticisms of ARUBA, the most relevant being the short follow-up of a life-long morbid condition. Other concerns include the slow patient recruitment, the high degree of selection bias, the lack of treatment standardization, and the lack of subgroup analysis to indicate which treatment modality was most dangerous. In the post-ARUBA era, more evidence has accumulated in ARUBA-eligible patients indicating that complications of embolization +/- radiosurgery remain high, up to 20% stroke and death Reference Singfer, Hemelsoet and Vanlangenhove66,Reference Mosimann and Chapot67 . Two surgical studies have obtained results quite different from ARUBA, prompting calls for a new RCT (BARBADOS) Reference Teo, St. George and Lawton68 .
There is some evidence that transvenous (TV) embolization can be effective, particularly for deep-seated and surgically inaccessible lesions Reference Fang, Byun, Liu, Krings, Pereira and Brinjikji69 . In a review of 8 series including 66 highly selected patients, complete occlusion was achieved in 96% with technical complications in 8% and good functional outcomes in 89%.
For unruptured bAVMs, the results of ARUBA, although flawed, must be heeded until better evidence is available. Although not a universal solution, observation and medical management are generally most appropriate, while active intervention should be considered in young patients with symptomatic AVMs. For ruptured bAVMs, multimodality therapy, depending on clinical features and local expertise, is usually the preferred approach, acknowledging that there are still significant risks with incomplete efficacy Reference Feghali and Huang70 .
Part VII: Dural Arteriovenous Fistulae
Dural arteriovenous fistulae (DAVFs) are enigmatic lesions, which are of uncertain origin, may be simple or complex, may represent a benign nuisance or lethal menace, and can be exceedingly difficult to treat. They represent 10%–15% of all intracranial vascular malformations and were mistakenly thought to represent AVMs rather than fistulae for many years. The most commonly used classification systems, Borden-Shucart and Cognard, are based on the pattern of venous outflow with the most dangerous being those with cortical venous drainage (CVD) (Figure 14). They can present with minimal symptomatology like tinnitus and headache, or with ICH and major neurologic deficits from venous hypertension affecting the brain. The annual risk of hemorrhage from the lower grade lesions is 1.5% or less, while with higher grade lesions, the annual risk can be up to 15%, with mortality of 10%. In patients who present with hemorrhage or aggressive neurological symptoms, the risk of recurrent bleed can be up to 35% Reference Reynolds, Lanzino and Zipfel71 .

Figure 14: (A) Left external carotid artery DSA, lateral view. Dural AVF of the left sigmoid sinus (arrow). (B) Unsubtracted lateral view with Onyx cast post-embolization. (C) Left external carotid artery DSA, lateral view post-embolization. Complete obliteration of AVF.
Endovascular techniques have been the mainstays of treatment for decades. The goal is to completely eliminate the arteriovenous shunt, using transarterial (TA) and/or TV routes. TA embolization is most effective when there are relatively few feeding arteries, or TV access is limited by the presence of a stenotic, compartmentalized, or isolated dural sinus or cortical vein. For the TA approach, distal catheterization of feeding vessels is essential in order for embolic material to penetrate the fistulous connection and proximal venous outlet. Onyx and the newer agents (Squid [Balt, Montmorency, France], PHIL [Microvention, Alison Viejo, CA]) are preferred, as they allow lengthy, more controlled injections with the ability to completely eliminate complex lesions via one arterial pedicle. Detachable tip microcatheters can facilitate long injections. Dual lumen balloon catheters and “pressure-cooker” techniques with two microcatheters and coils can prevent reflux. N-BCA is more difficult to control, and it is easier to occlude the venous outlet while the fistula is still patent. Multiple injections are often needed, and there is a higher risk of cranial nerve injury with dangerous vascular anastomoses. Partial embolization does not lower the hemorrhage risk and may in fact make lesions more dangerous by the rerouting of venous drainage. A novel use of flow diverters for cavernous sinus lesions has been described Reference Baharvahdat, Ooi, Kim, Mowla, Coon and Colby72 .
TV approaches are particularly effective when there are innumerable small arterial feeders, when venous access is relatively straightforward, and when a diseased sinus does not drain normal brain. TV embolization, using coils with or without liquid embolic agents, can be particularly useful for DAVFs of the cavernous and sigmoid sinuses. Innovative remodeling and tunneling strategies have been described in dural sinuses for complex lesions Reference Baharvahdat, Ooi, Kim, Mowla, Coon and Colby72 .
Complete cure rates of up to 80% using Onyx via the TA approach have been reported. Complete cures of 71%–87.5% have been described for TV therapies, with complications in 4%–7% Reference Gandhi, Chen, Pearl, Huang, Gemmete and Kathuria73 . Surgical options, including coagulation of feeders, venous disconnection, and sinus skeletonization, are pursued when EVT fails or is incomplete. Efficacy is good, but morbidity can be higher (10%). Radiosurgery can be employed when other options are exhausted and cure rates of 50%–93% have been reported, particularly for lesions at the anterior skull base, but there is a long latency period, which can be dangerous for lesions with CVD Reference Fang, Byun, Liu, Krings, Pereira and Brinjikji69 .
Part VIII: Cerebral Vasospasm
Vasospasm with delayed cerebral ischemia remains the primary cause of morbidity and mortality in those patients who survive an initial subarachnoid hemorrhage (SAH). Angiographic vasospasm can be seen in up to 70% of SAH patients, with ⅓ to ½ becoming symptomatic. Estimates of long-term functional dependency range from 20% to 50%, with mortality up to 50%.
Systemic medical therapy, including “Triple-H” (Hypertension, Hypervolemia, and Hemodilution) and oral nimodipine, is the first line of treatment, although evidence for efficacy is mixed. EVT began in 1984 when Zubkov et al. described results in 33 patients treated with balloon angioplasty Reference Zubkov, Nikiforov and Shustin74 . Since that time, both transluminal balloon angioplasty (TBA) and a variety of IA drug infusions have been used with mixed results. Despite the prevalence and clinical significance of cerebral vasospasm, there is no RCT evidence for the superiority of EVT to standard medical care.
TBA results in immediate vascular dilatation but causes intimal damage, endothelial denudation, flattening of the internal elastic lamina, and stretching of smooth muscle. It is limited to proximal intracranial vessels. Retreatment is usually not necessary, but major complications, including usually fatal vessel rupture, can be seen in up to 5% of patients Reference Hoh and Ogilvy75 . Prophylactic TBA has been shown to be of no value Reference Zwienenberg-Lee, Hartman and Rudisill76 . Although immediate angiographic improvement may be impressive, there is still no Level 1 evidence for clinical efficacy Reference Pandey, Elias, Chaudary, Thompson and Gemmete77 .
Papaverine, an opiate alkaloid antispasmodic, was the first drug used for direct IA infusion in 1992. It interferes with calcium ion channels in smooth muscle, and immediate improvement in vessel diameter can be seen in up to 80% of patients (Figure 15). Clinical improvement, however, is much lower (26%), and angiographic changes are temporary with retreatment needed in at least 34% Reference Park, Kim and Kang78,Reference Sokolowski, Chen and Ding79 . A variety of other drugs have been used, and the most common being calcium channel blockers such as verapamil, nimodipine, and nicardipine (Figure 16). Other agents such as fasudil and milrinone have also been utilized, with no clear advantage of any medication Reference Sokolowski, Chen and Ding79,Reference Venkatraman, Khawaja and Gupta80 . A recent meta-analysis of 55 observational IA vasodilator studies showed that over 90% demonstrated immediate angiographic improvement, but only 60% showed clinical improvement and mortality was 9%–11% Reference Venkatraman, Khawaja and Gupta80 . The discordance between radiographic and clinical outcomes indicates that metabolic and circulatory factors are likely at least partially independent of vessel diameter.

Figure 15: (A). Right common carotid artery DSA, AP view. Severe right MCA vasospasm post-SAH (arrow). (B) Right common carotid artery DSA, AP view. Post intra-arterial infusion of papaverine.

Figure 16: (A) Right common carotid artery DSA post-SAH. Moderate-to-severe vasospasm in MCA. (B) DSA post-intra-arterial verapamil. Mild improvement of vasospasm.
The general consensus for IA vasospasm treatments seems to be that earlier initiation is more likely to have clinical benefit, but that often-impressive angiographic changes have yet to guarantee good neurological outcomes.
Part IX: Spinal Vascular Malformations
Djindjian in France and Di Chiro in the US pioneered spinal angiography in the 1960s. This allowed precise definition and eventual EVT of spinal vascular malformations. They represent a diverse, relatively rare group of disorders (3%–4% of all spinal lesions) with a number of classification systems. There are two main groups: spinal AV fistulae, with a direct shunt between arteries and veins, and spinal AVMs, in which there is a definite vascular nidus intervening between arteries and veins. Dural AV fistulae are most common, accounting for approximately 70% of all spinal vascular lesions. They were incorrectly called AVMs for decades, leading to surgical excision of venous networks and frequent spinal infarction. They are most common in males, present in the 6th decade, are usually solitary and thoracic or lumbar in location. They rarely bleed, but present with neurological symptoms related to venous hypertension Reference Chaudhary, Pandey and Gemmete81 . Spinal angiography is essential for precise localization of arterial inflow and venous outflow.
Embolization is most appropriate for symptomatic spinal AVMs, due to the high risk of surgical resection, particularly with intramedullary lesions (15%–28%). Liquid embolics such as n-BCA and Onyx are the preferred agents and are often used as a primary treatment or less commonly as an adjunct to surgery. Several series have reported > 50% decrease in nidus size in up to 86% of patients, with approximately 13% permanent morbidity. Even partial embolization of intramedullary lesions can improve neurological function Reference Chaudhary, Pandey and Gemmete81 .
EVT of dural AVFs began in 1968 with a total obliteration reported in 70%–90% of cases. It can, however, be technically challenging to avoid proximal arterial occlusion, to access multiple feeding arteries, and to completely obliterate the shunt and proximal venous outlet. Recanalization in these circumstances is frequent. Surgical excision can be straightforward, with an obliteration rate of 98% and is now the preferred therapeutic option Reference Steinmetz, Chow and Krishnaney82 . Long-term follow-up reveals inconsistent results regarding neurological recovery Reference Chaudhary, Pandey and Gemmete81 .
Part X: Percutaneous Sclerotherapy of Head and Neck Venous and Lymphatic Malformations
Venous (VM) and lymphatic malformations (LMs) are developmental, low-flow vascular malformations most commonly seen in the head and neck. They consist of aberrant venous and lymphatic channels with disorganized endothelium, which may consist of numerous small (microcystic) or large (macrocystic, > 2 cm diameter) cystic cavities. Presentation is in the perinatal period or in childhood, and the natural history is variable. Many will involute spontaneously or remain a purely cosmetic deformity. They can slowly increase in size, they can become infected and cause pain, and they can compress the aerodigestive tract, visual, and auditory structures. MRI is the imaging modality of choice. Surgical resection is difficult as the lesions tend not to be encapsulated and frequently interdigitate with normal structures.
Percutaneous sclerotherapy has been the preferred therapeutic option, using a variety of agents that collectively result in low-grade inflammation with eventual thrombosis and fibrosis. Absolute ethanol was the first and remains possibly the most effective sclerosant agent, but it is painful and toxic with up to 50% of patients suffering minor complications. The first line of therapy is now usually sotradecol (sodium tetradecyl sulfate), a less toxic detergent. The glycopeptide antibiotic and chemotherapeutic agent bleomycin is also commonly used for VMs (Figure 17), and the antibiotic doxycycline is often the first line of treatment for LMs.

Figure 17: (A) MRI, coronal FSE right orbit. Complex, multiloculated venous malformation (arrows). (B) MRI, coronal FSE at 6 months post-percutaneous sclerotherapy with bleomycin. Complete obliteration of the venous malformation.
There is no RCT evidence for clinical efficacy, and clinical response is based on nonrandomized and single-center case series (Level IIa, Grade B). Improvement in lesion size is seen in 75%–95% of patients within 8 weeks. Most patients, however, will need multiple treatments for maximum clinical benefit. Mild complications such as skin discoloration and ulceration are seen in 10%–12% of patients. Severe complications, such as skin necrosis, nerve injury, or systemic effects, are rare, and most common when ethanol is used as the sclerosant Reference Heit, Do and Prestigiacomo83 .
Part XI: Meningiomas and Tumor Embolization
Meningiomas are highly vascularized tumors that are particularly suited to preoperative embolization because of their primary supply from branches of the external carotid artery (ECA). Advantages include decreased operative time and blood loss with alterations in tumor consistency from devascularization and necrosis, all leading to more manageable surgery and potential for more complete resection. Since the initial report by Manelfe in 1973, there have been numerous retrospective case series published in which many agents, including PVA particles, microspheres, coils, fibrin glue, n-BCA, and Onyx, have been utilized to facilitate surgery. Despite the decades of experience, there is still no RCT evidence of efficacy or consensus regarding indications for preoperative embolization. Many recommendations are based on subjective surgical impressions. Objective data are difficult to accumulate due to the heterogeneity of patient and tumor characteristics, with larger and more complex lesions often being selected for embolization.
Embolization success rates are excellent (91%–100%), particularly with convexity lesions supplied by the middle meningeal artery (MMA). Complication rates vary from 0% to 9% Reference Venkatraman, Khawaja and Gupta80 , most commonly due to cranial nerve injury in skull base lesions, intra-tumoral hemorrhage, mass effect from tumor swelling, or dangerous vascular anastomoses. Most series recommend surgery within 1 week of embolization to prevent vascular recanalization or collateral recruitment, although some recommend waiting for delayed tumor necrosis. There have been reports of intraoperative embolization when distal vascular access is challenging. General recommendations are that preoperative embolization is most valuable for larger tumors (>3–4 cm), with at least 50% supply from the ECA, vascular tumors with deep arterial supply, which may be difficult for surgical control, tumors in eloquent areas and those without major calcification Reference Shah, Choudhri, Jung and Li84,Reference Raper, Starke and Henderson85 .
Many of the principles of preoperative embolization apply in a similar fashion to other vascular tumors of the brain, head, and neck, including metastatic lesions, hemangioblastomas, juvenile nasopharyngeal angiofibromas (JNAs), glomus jugulare, and other paragangliomas Reference Duffis, Gandhi and Prestigiacomo86 . Direct puncture techniques using Onyx have been useful for some head and neck tumors. Individual patient and tumor characteristics will determine the feasibility and utility of preoperative therapy.
Part XII: Venous Sinus Stenting for Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension (IIH) is a condition consisting of papilledema and headaches, sometimes with tinnitus and is most often found in overweight females of childbearing age. The underlying pathophysiology is uncertain. Imaging often demonstrates stenosis of the transverse dural venous sinus near the junction with the sigmoid sinus. Although the mechanism is unclear, most authors favor external compression by congested brain and cerebrospinal fluid (CSF) spaces. There have been many reports of significant improvement in medically intractable symptoms following endovascular venous sinus stenting. The largest meta-analysis Reference Nicholson, Brinjikji and Radovanovic87 of 474 patients showed that there was an improvement in papilledema in 93.7%, pulsatile tinnitus in 90.3%, and headache in 79.6%. This compares to rates of improvement in headache of 80% and papilledema of 70% with CSF diversion. The overall recurrence rate was 9.8%, with 12.4% needing a second stent and 3% requiring CSF diversion. Complications including subdural and subarachnoid hemorrhage occurred in 1.9% of patients, and there were no fatalities. Although there is still no RCT evidence of efficacy compared to medical therapies, the authors conclude that venous stenting an effective treatment for the major morbidities of IIH with an excellent safety profile.
Part XIII: Inferior Petrosal Sinus Sampling for Cushing’s Disease
Approximately 70% of all Cushing’s disease results from pituitary microadenomas. MRI is only 40%–50% accurate in detecting the often-tiny basophilic adenomas responsible for excess adrenocorticotrophic hormone (ACTH) secretion. Detection of a small lesion within the gland does not definitively identify the source, as 10%–20% of the population can harbor “incidentalomas” of no functional significance. Inferior petrosal sinus sampling (IPSS) has been the gold standard for confirming the central origin of ACTH in Cushing’s disease since the first description of unilateral sinus sampling in 1977 and bilateral sampling in the 1980s Reference Oldfield, Doppman and Nieman88 . Technical success rates are over 90%, the sensitivity is approximately 96%, and specificity 100% for central localization, with approximately 84% accuracy for lateralization within the pituitary gland. The technique has not changed since the original descriptions, with no need to place microcatheters in the cavernous sinuses. Complications are rare (<1%) although brainstem infarction due to venous obstruction has been described Reference Lad, Patil, Laws and Katznelson89 .
Part XIV: Vertebroplasty and Spinal Interventions
There have been thousands of publications describing the results of percutaneous vertebral augmentation using polymethylmethacrylate (PMMA) for osteoporotic compression fractures since the original descriptions in the 1970s. Anecdotal evidence for efficacy was convincing, and there was a rapid evolution of a major medical industry dedicated to this patient population. The publication of two RCTs in 2009, showing no benefit of the procedure over placebo, halted this juggernaut Reference Buchbinder, Osborne and Ebeling90,Reference Kallmes, Comstock and Heagerty91 . Although briefly resurrected in 2016 for acute fractures Reference Hirsch and Chandra92 , the recent VERTOS IV Trial Reference Firanescu, de Vries and Lodder93 confirmed the absence of clinical benefit during 12 months of follow-up. Pain relief can be dramatic; however, most investigators attribute this to the placebo effect.
Between 5% and 10% of all cancer patients develop spinal metastases. Vertebroplasty and kyphoplasty (in which a balloon is used to maximize PMMA deposition and restore vertebral height) have been shown to significantly improve pain and disability in patients with cancer-related vertebral compression fractures. A review of over 3400 patients with pathologic fractures showed that the procedures were effective as measured by the Visual Analog Score, Oswestry Disability Index, and Karnofsky Performance Score. Complication rates were 1%, most often due to cement leakage Reference Sorensen, Kirkegaard, Carreon, Rousing and Andersen94 .
Spontaneous intracranial hypotension is an increasingly recognized cause of headache (usually orthostatic and often debilitating) and other more serious sequelae such as dementia, quadriplegia, and coma. It is most commonly caused by a spontaneous spinal CSF leak. The site of leakage may be exceedingly difficult to find, often requiring multiple imaging modalities such as CT, MRI, and dynamic digital subtraction myelography Reference Schievink, Maya, Louy, Moser and Tourje95 . The condition may resolve with epidural blood patching but will sometimes require more invasive therapies. If the site has been identified, targeted percutaneous, CT-guided epidural injections using autologous blood and/or fibrin glue can successfully seal the leak. If the site has not been found, large-volume injections of up to 80 ccs of autologous blood, delivered through a single site epidural catheter, have been shown to be useful Reference Griauzde, Gemmete, Chaudhary, Wilson and Pandey96 .
Neuroradiologists often perform percutaneous spinal injections for pain management, particularly facet blocks, transforaminal, and interlaminar epidural steroid injections. Anecdotal evidence for their efficacy is impressive, demand is overwhelming, and remuneration can be lucrative. There is, however, no RCT evidence for their benefit over placebo, minor complications are not uncommon Reference Manchikanti, Malla, Wargo, Cash, Pampati and Fellows97 , and the long-term effects of intra-articular corticosteroids on cartilage are not insignificant Reference Wernecke, Braun and Dragoo98 .
Part XV: Reflections
In 1984, Pierre Lasjaunias, an INR visionary and international educator, stated, “We can do anything.” This new specialty allowed unique access to and treatment of many cerebrovascular lesions. Endovascular therapies have since revolutionized the treatment of cerebrovascular disorders, particularly AIS, aneurysms, and fistulae. Imaging and clinical results can be spectacular. Neurointerventional practitioners are now essential for stroke and cerebrovascular centers worldwide. The impact has spread to the large populations of India, China, and Southeast Asia, and the opportunities for international collaboration are unlimited. The future of INR is bright, bringing promise of biological modulation of implanted devices, robotic interventions, and artificial intelligence applications.
In many areas, the results may be impressive, but RCT evidence of efficacy over surgery or less invasive therapies is lacking. Treatment of asymptomatic patients, particularly cerebral aneurysms and extracranial carotid atherosclerosis, remains controversial. A specialty dependent on expensive technology must critically evaluate new devices to avoid industry-driven practice. There must be careful, ongoing research focusing on clinical indications, outcomes, complications of treatments, and cost-effectiveness. Affordability is key in order for the technology to achieve maximum benefit. Close collaboration with allied specialties, particularly neurosurgery and neurology, is essential to insure appropriateness of intervention.
INR can do a great deal, but not everything. It is often not a question of whether something can be done, but rather, should it be done. In the words of John Hunter, considered by many to be the father of experimental and modern surgery (Figure 18):
No operation should be carried out unless absolutely necessary…. nor should a surgeon operate unless he would undergo the same operation himself under similar circumstances.

Figure 18: John Hunter, 1728–1793, by Sir Joshua Reynolds.
Acknowledgments
The authors would like to acknowledge and thank Drs. Allan Fox, Fernando Vinuela, Jennifer Mandzia, David Spence, Vladimir Hachinski, Rob Hammond, Karel terBrugge, Mel Boulton, and Anne Abbott for their valued input to this manuscript.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
DM Pelz performed the literature review and is the primary author of this manuscript.
SP Lownie provided invaluable editorial assistance for definitive revision of the manuscript.
Drs. MS Mayich, SK Pandey, and M Sharma provided case material for figures and provided editorial assistance for revision of the manuscript.




















