Introduction
Scorpion and snake envenomation are common causes of morbidity and mortality in the developing world. Manifestations following a snake bite and a scorpion sting are diverse. Neurological complications following snake and scorpion bite are protean. The sting of Mesobuthus tamulus (Indian red scorpion) and snakes of family Viperidae are known to cause cerebrovascular complications in rare cases. Literature regarding patterns of cerebrovascular injury (CVI) and outcomes among these patients is scarce. The objective of this study was to assess the demographic characteristics, clinical profile, brain imaging findings and details of vascular territory involvement among patients admitted with CVI following scorpion and snake envenomation, in a tertiary care center in South India. We also aimed to study the outcomes in terms of mortality in patients admitted with CVI following scorpion and snake envenomation.
Methodology
This study was conducted in Christian Medical College, Vellore, which is a 2700-bedded tertiary care health center in South India. This retrospective case series included patients seen in the General Medicine, Radiology Departments of Christian Medical College. All patients with a proven diagnosis of scorpion and snake envenomation were included in the study if they had (1) new-onset focal neurological deficit after the initial neurological manifestations subsided, (2) persistent declining in sensorium after 24 hours of admission despite adequate medical management and the (3) persistent presence of neurological deficits. If the patient fulfilled the inclusion criteria, neuroimaging was performed. All patients with scorpion envenomation between May, 2005 and August, 2016 (11 years) were retrospectively enrolled in the study. Similarly, patients with snake envenomation between May, 2014 and August, 2016 (2 years) were enrolled retrospectively. Patients who did not have central nervous system (CNS) imaging were excluded from the study.
A total of 196 and 55 patients with snake envenomation and scorpion sting, respectively, were included in the study. Among these patients with snake and scorpion envenomation, five patients each satisfied inclusion criteria and were recruited for analysis. After recruitment, the details of demography, clinical features, laboratory features, imaging features, details of treatment and outcome were obtained from the hospital electronic medical records. The neuroimaging details on the pattern of injury, presence and characterization of infarcts were studied by an independent neuro-radiologist who was unaware of the patient outcome. The details of the site, size, number and territory of infarcts were obtained by the radiologist. Data were entered in Microsoft Excel version 9, and data analysis was performed using statistical package for social science version 16. The principal outcome that was studied was death. Mortality was ascertained at the time of discharge. We searched the standard database including MEDLINE and Google Scholar for articles mentioning cerebrovascular accident following snake and scorpion envenomation written in the English language after the year of 2000, including patients older than 16 years, providing appropriate CNS images. On reviewing the literature, we identified 32 and 29 cases of CVI following snake and scorpion envenomation, respectively, and analyzed the finding of all these cases together. In all, 35 age-matched controls with CVI and without envenomation were included in order to compare the differences in topography in CVI following snake/scorpion envenomation versus the age-matched controls.
Results
In all, 9% (5 of 55) and 2% (5 of 196) of patients with scorpion sting and snake envenomation fulfilled the inclusion criteria and required neuroimaging. Among the five patients who fulfilled the inclusion criteria in both the groups, three had CVI as seen in imaging, respectively (n=6). All six patients were male. The mean age of the patients with scorpion sting and snake envenomation was 40.6 and 36 years, respectively. Local pain, profuse sweating, palpitations and high blood pressure indicating severe toxemia were present in all the patients with scorpion sting. Similarly, all the three patients of Russell’s viper envenomation showed the presence of local swelling, local and systemic bleeding and respiratory failure. While two patients had infarcts, one had a hemorrhage in either group. The mean time to onset of symptoms following the scorpion sting was 1.6 days, as compared with 4 days following snake bite. The mean duration of in-patient stay following the envenomation was 5 days for scorpion stings and 16 days for snake bites. All patients with scorpion sting were discharged in stable condition and two patients with snake envenomation were discharged in stable condition.
A total of 32 patients with scorpion sting and 35 patients with snake envenomation were included in the review, as shown in Table 1. Mean ages of patients with snake and scorpion envenomation were 43 and 33 years, respectively. There was a male preponderance among both the groups. Clinical features of depressed sensorium and hemiparesis were present in more than one-third of the patients with snake envenomation. Hemiparesis was present in more than half of the patients, and depressed sensorium was present in one-third of the patients with scorpion envenomation. CT brain was the most common neuroimaging performed for 54% and 81% of patients with snake and scorpion envenomation, respectively. Infarcts were the most common patterns of CVI and were seen in 88% and 53% with snake and scorpion envenomation, respectively. Among these, 68% of patients with snake envenomation and 47% of patients with scorpion sting had multiple infarcts. More than one infarct was defined as multiple infarcts. In all, 48.5% of patients with snake envenomation and 28.5% of patients with scorpion sting had infarcts bilaterally. Among the controls, 26 (74%) had infarcts, seven (20%) had hemorrhages and two (6%) had hemorrhagic infarcts. Among the controls, 13 (50%) infarcts were multiple and six (23%) were bilateral. Hemorrhages were more among patients with scorpion sting (41% vs. 6%). In all, 92% of patients with hemorrhage had single and unilateral involvement. Among the controls, all bleeds were single and unilateral. In the patients with scorpion sting, the most common sites of hemorrhage were capsuloganglionic region, similar to the controls, as shown in Table 2. The most common sites of infarcts following both the envenomations were parieto-occipital, cerebellar and capsuloganglionic and frontal region, respectively (Images 1 and 2). These were also similar to the topography of infarcts in age-matched controls (Table 2). Mortality in patients with cerebrovascular accidents following snake envenomation was 8%, as compared with 28% in patients with scorpion envenomation and 5.7% in age-matched controls.
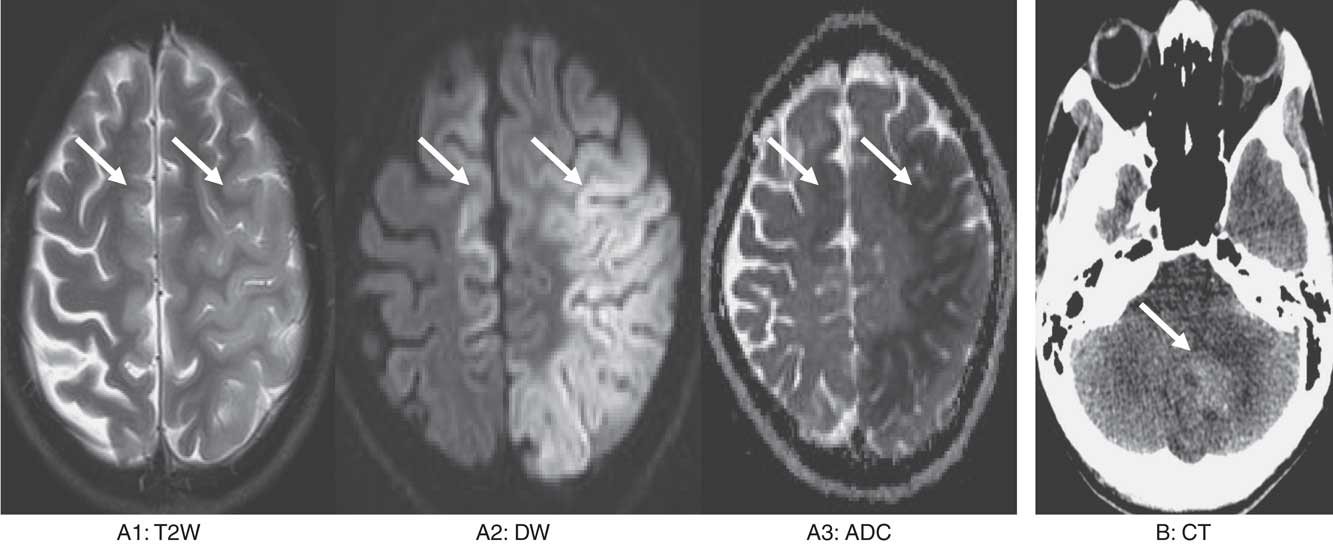
Image 1 Image series (A) a 36-year-old man with snake bite. T2 weighted (T2W) shows swelling and hyperintensity in left middle cerebral artery and right anterior cerebral artery territory with associated diffusion restriction suggestive of acute infarct. (B) A 38-year-old man with snake bite. Non-contrast CT shows acute hematoma in the left cerebellar hemisphere with surrounding edema.
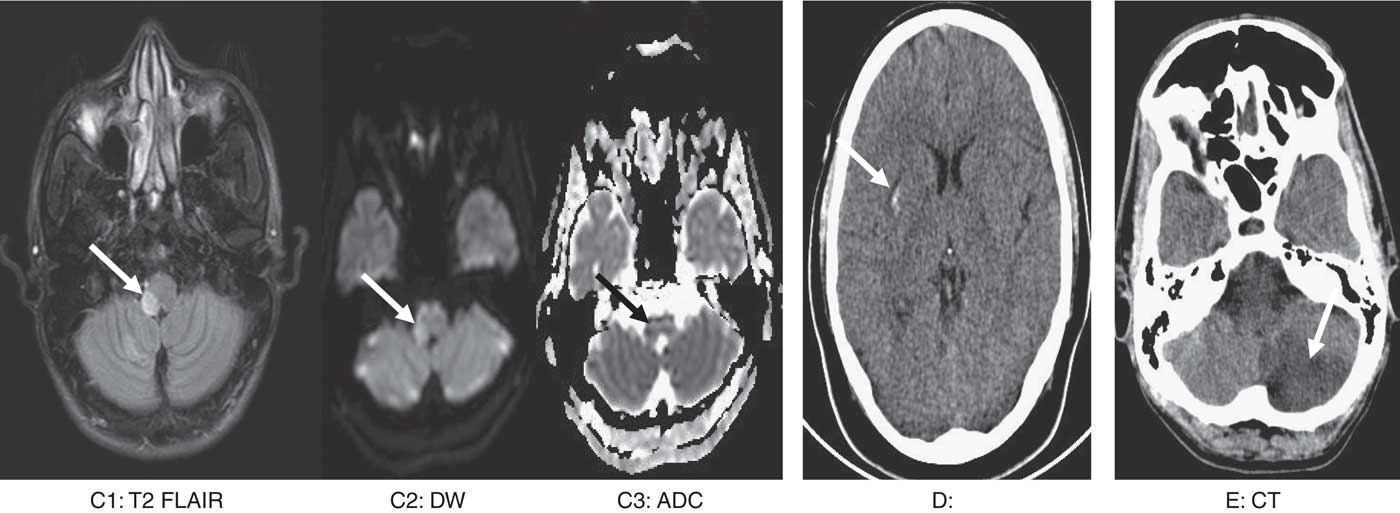
Image 2 Image series (C) a 45-year-old lady with scorpion sting. T2 flair image shows hyperintensity and expansion. Corresponding area shows diffusion restriction suggestive of acute right lateral medullary infarct. (D) A 29-year-old man with scorpion sting. Non-contrast CT brain show acute hemorrhage in the right putamen. (E) A 47-year-old man with scorpion sting. Non-contrast CT brain shows acute infarct in the left posterior inferior cerebellar artery territory.
Table 1 Details of previously reported cases of cerebrovascular injury (CVI) in patients with snake envenomation and scorpion sting
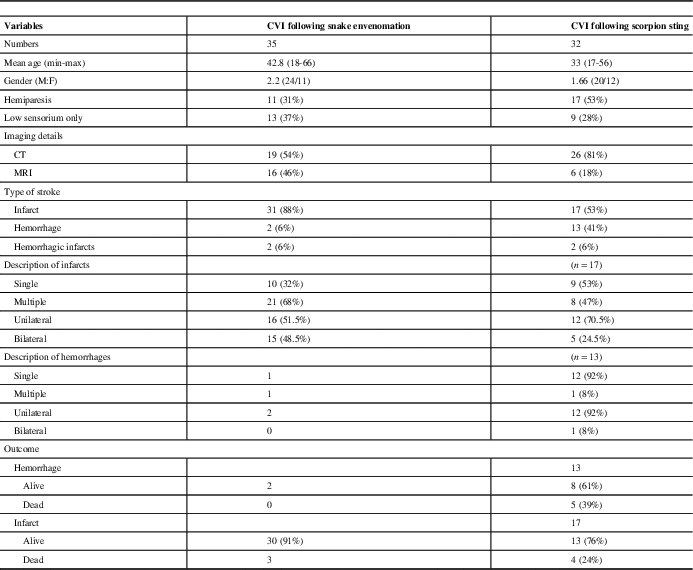
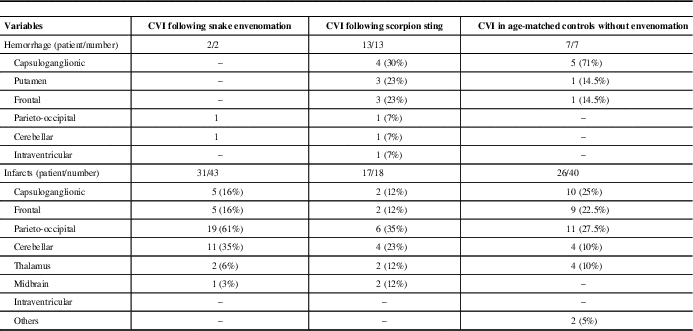
Table 2 Location of hemorrhage and infarcts following snake and scorpion envenomation and in age-matched patients with cerebrovascular injury (CVI) without envenomation
Discussion
Envenomation is common in developing countries, with more than 50,000 and 2600 reports of mortality following snake and scorpion envenomations being reported per year.Reference Elkhayari, Hachimi, Ziadi and Abdenasser Samkaoui 1 , Reference Gawarammana, Mendis and Jeganathan 2 Along with the classical neurological manifestations of external ophthalmoplegia, ptosis and respiratory failure, these can also have multiple other systemic manifestations. An uncommon neurological manifestation following these envenomations is CVI.Reference Lee, Hwang, Bae and Kwon 3 , Reference Nagaraja, Verma, Taly, Kumar and Jayakumar 4 Reports of CVI are seen in 2.6%-4% of patients with snake and scorpion envenomations.Reference Del Brutto and Del Brutto 5 , Reference Narang, Paleti, Asad and Samina 6 Snakes of Viperidae family including Russel’s viper, Indian green tree viper, Korean viper, species of other pit vipers such as Cerastes, Bothrops and Crotalids are reported to cause complications related to cerebrovascular involvement.Reference Mahale, Mehta, Javali and Srinivasa 7 – Reference Paul, Paul and Puri 9 Among all the viper envenomations, reports of CVI are the highest among Russel’s viper envenomation.Reference Subasinghe, Sarathchandra, Kandeepan and Kulatunga 10 – Reference Gouda, Pandit, Seshadri, Valsalan and Vikas 12 Similarly, scorpions such as M. tamulus and Tityus serrulatus have been reported to cause cerebrovascular involvement.Reference Eze, Onwuekwe and Ekenze 13 – Reference Jain, Indurkar, Kastwar and Malviya 15 Various mechanisms have been postulated in causing vascular injury. Proteases and hemorragins cause direct damage to the vascular endothelium. Various toxins present in viper venom have both pro-coagulant and anti-coagulant effects. Toxins with pro-coagulant effect are cerastobin, cerastocytin, cerastotin, afaacytin and factor IVa. These toxins have properties of platelet aggregation, thrombin-like activity, pro-coagulant metalloproteases and activators of factor V and X, which are instrumental in the activation of the coagulation cascade. The other mechanisms of vascular injury are toxin-induced vasospasm or toxic vasculitis, toxin-induced disseminated intravascular coagulation and hypoperfusion due to hypovolemia and hypotension.Reference Rebahi, Nejmi, Abouelhassan, Hasni and Samkaoui 16 – Reference Bush, Mooy and Phan 18 Mechanisms that have been postulated in patients with scorpion stings are (i) the presence of myocarditis leading to rhythm abnormality and embolic strokes, (ii) autonomic storm causing extremes of blood pressure, (iii) severe cerebral vessel vasospam owing to catecholamine excess and (iv) toxin-induced endothelial injury and vasculitis.Reference Sarkar, Bhattacharya and Paswan 19 – Reference Udayakumar, Rajendiran and Srinivasan 21
At the time of initial presentation, these patients might have no neurological symptoms or depressed sensorium without any signs.Reference Gadwalkar, Bushan, Pramod, Gouda and Kumar 22 , Reference Cañas 23 In a smaller subgroup of patients, cerebrovascular involvement should be considered in case of (1) persistence of depressed sensorium, initial neurological deficits and new-onset focal deficit.Reference Ittyachen and Jose 24 – Reference Sengupta, Dhanapal, Ravindran and Devi 26 Persistently depressed sensorium is the most common neurological manifestation reported in the literature, as shown in Table 1. The other clinical manifestations that have been reported are new-onset generalized tonic–clonic seizures, varying motor weakness including hemiplegia, presence of visual field deficit and blindness, giddiness and dysarthria.Reference Raichur, Magar, Wari and Chandragouda 27 – Reference Gupta, Tewari and Nair 29
Both CT and MRI have been used for confirmation of CVI among these patients. CT brain was performed for 75% of patients with scorpion sting and 53% of patients with snake envenomation in our review. Although CT is adequate to establish the diagnosis, an MRI with diffusion restriction is ideal in differentiating dilated venules and artifacts, which could be mistaken for infarcts.Reference Mishra, Arvind and Muliyil 30 In both, infarcts and hemorrhages can be seen, with the former being common. Infarcts tend to be bilateral and multiple. Infarcts following both the envenomations were predominant in parieto-occipital, cerebellar and capsuloganglionic regions, respectively (Images 1 and 2). Involvement of bilateral anterior cerebral artery, middle cerebral artery and the posterior cerebral artery is common, with the involvement of bilateral posterior cerebral artery reported to be most common.Reference Kumar, Babu and Agrawal 31 – Reference Thacker, Lal and Misra 39 The uncommon neurological involvements that have been reported are the presence of thalamic infarct, lateral medullary syndrome and brainstem infarcts.Reference Ittyachen and Jose 24 , Reference Kumar, Babu and Agrawal 31 , Reference Fernández-Bouzas, Morales-Reséndiz, Llamas-Ibarra, Martínez-López and Ballesteros-Maresma 35 , Reference Thomas, George, Mishra, Mannam and Ramya 40 Hemorrhages tend to be unilateral and single. Common sites of hemorrhage are capsuloganglionic region, cortical and cerebellar, respectively (Table 2) (Images 1 and 2). Patients can also present with coexistent infarcts and hemorrhages, which was 6% in this review.Reference Namal Rathnayaka, Kularatne, Kumarasinghe, Ranaweera and Nishanthi Ranathunga 11 , Reference Boviatsis, Kouyialis, Papatheodorou, Gavra, Korfias and Sakas 32
All patients reviewed had features of severe envenomation at the time of admission. All these patients were treated medically and received blood products, anti-snake venom, an alpha-blocker (in case of scorpion sting) and optimal supportive care. Reported mortality of patients with cerebrovascular involvement following scorpion and snake envenomation is diverse, as compared with 8% following snake and 28% in scorpion envenomation. As most of these patients have evidence of venom-induced severe systemic toxicity such as shock, rhabdomyolysis, acute renal failure and coagulopathy, mortality among these patients cannot be solely attributed to the CVI.Reference Thomas, George, Mishra, Mannam and Ramya 40 , Reference Chandrashekar, Anikethana and Kalinga 41 Future prospective studies with early diagnosis and prompt treatment can result in further reduction of mortality.
Most patients in this review had been recruited retrospectively, and hence detailed cardiac, autoimmune and thrombotic work-up had not been performed. No details were available on baseline National Institutes of Health Stroke Scale or Modified Rankin Scale of these patients. Similarly, we did not have details of validated functional neurological outcome including details on long-term follow-up. Variability in the severity of the toxidrome, delay in the presentation of the patient to a health care center and protocols of treatment could have had an impact on the events and outcome.
Conclusions
In conclusion, CVIs are uncommon neurological manifestations following scorpion and snake envenomation. These are more common in younger age group males without risk factors for stroke. This should be suspected in patients with persistently depressed sensorium and new-onset focal neurological deficits. In CNS imaging, infarcts are more common than bleeds. With optimal supportive medical care, mortality and morbidity are reduced. Further studies are required to understand the mechanism, risk factors and long-term neurological outcomes of these patients
Acknowledgments
The authors would like to acknowledge Dr. Suniti Mani, Dr. Anu Anna George and Dr. Karthik G.
Statement of Authorship
All authors certify that they have participated sufficiently in the preparation of this manuscript.
Disclosure
AM, AB, GA, HV, TG and RI have nothing to disclose.






