Adipokine leptin regulates energy homeostasis and immune and metabolic functions(Reference Dardeno, Chou and Moon1). Its concentration reflects the amount of body fat, being typically highly elevated in overweight individuals(Reference Dardeno, Chou and Moon1) who concomitantly manifest leptin resistance(Reference Myers, Leibel and Seeley2, Reference Deng and Scherer3). Elevated leptin concentrations have also been detected in patients with cardiovascular and metabolic diseases(Reference Deng and Scherer3–Reference Kajikawa, Ikeda and Takemoto5) as well as in asthmatic and allergic individuals(Reference Mai, Chen and Krewski6–Reference Quek, Sun and Ng8), suggesting leptin's contribution in the onset or progression of these diseases. During pregnancy, highly elevated leptin concentrations are associated with aberrations in maternal metabolism(Reference Vahamiko, Isolauri and Pesonen9) and, thereafter, an increased risk of gestational diabetes and pregnancy-induced hypertension(Reference Heerwagen, Miller and Barbour10, Reference Butte, Hopkinson and Mehta11). When taking into account the fact that an adverse maternal metabolic status, including overweight and elevated leptin concentration, has been suggested to programme offspring's later risk for obesity and metabolic and cardiovascular disorders(Reference Fraser, Tilling and Macdonald-Wallis12, Reference Djiane and Attig13), it may be assumed that controlling the pregnancy-related increase in leptin concentrations may provide clinically significant health benefits for both women and their infants.
Factors determining leptin concentrations during pregnancy and in early infancy are poorly known. Nevertheless, dietary composition, in particular the amount and quality of carbohydrates, fibre and fat(Reference Tighe, Duthie and Vaughan14–Reference Malik, Popkin and Bray16), as well as the use of probiotic bacteria(Reference Laitinen, Poussa and Isolauri17–Reference Chapman, Gibson and Rowland19), has been suggested to modify other inflammatory markers and the risk of metabolic and immunological diseases. Therefore, we aimed here to evaluate whether dietary counselling, probiotic supplementation, dietary intake or maternal characteristics during pregnancy would have an impact on serum leptin concentrations in women, cord blood or in children.
Subjects and methods
Subjects and study design
The study population comprised pregnant women participating in an ongoing, prospective, randomised mother and infant nutrition guidance and probiotic study(Reference Piirainen, Isolauri and Lagstrom20, Reference Aaltonen, Ojala and Laitinen21). Recruitment took place during their first visit to maternal welfare clinics in South-West Finland. Criteria for inclusion were early pregnancy ( < 17 weeks) and allergy in the family (mother, father or sibling of the unborn child) and for exclusion chronic diseases such as diabetes or coeliac disease. A total of 256 subjects were recruited and randomised into three groups: a dietary counselling group with probiotic supplementation (n 85) or with placebo (n 86) and control group (n 85) with placebo. Women in the dietary counselling groups received individualised nutritional advice during the study visits and food products supporting the achievement of the recommended diet. In addition, they received in a double-blind manner either probiotic capsules containing Lactobacillus rhamnosus GG (American Type Culture Collection 53 103; Valio Limited) and Bifidobacterium lactis Bb12 (Chr. Hansen), 109 colony-forming units/d each, or placebo capsules that contained microcrystalline cellulose and dextrose anhydrate but no nutrients. Women in the control group received placebo capsules in a single-blind manner. Randomisation was based on a randomisation list, drawn up by a statistician, who was not involved in the study visit. All women received health and dietary counselling in maternal welfare clinics according to a national programme. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and we certify that all other applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed throughout this research. The study was approved by the Ethical Committee of the Hospital District of South-West Finland. Written informed consent was obtained from all the women involved. The present study is registered as a clinical study (NCT00167000; section 3, http://www.clinicaltrials.gov).
The participants visited the study clinic at the first (mean 14, range 7–18 weeks), second (mean 24, range 20–27 weeks) and third (mean 34, range 30–37 weeks) trimesters of pregnancy, and, with their infants, at 1 month postpartum (mean 1·3 months, range 0·03–3·6 months).
Dietary counselling and food records
Dietary counselling was aimed at affecting the type of fat used and to increase the amount of fibre in the women's diet as described in detail elsewhere(Reference Piirainen, Isolauri and Lagstrom20). The consumption of vegetables, fruits, whole-grain bread, cereals, leaner meat products, low-fat cheese and milk products as well as the use of vegetable oils and margarines was encouraged. Fish was recommended as one of the main meals twice per week. The achievement of an appropriate diet was supported by providing the mother with conventional food products available on the market with favourable fat and fibre content (e.g. rapeseed oil-based spreads, salad dressing and fibre-enriched pasta, muesli and cereals) for use at home. Women were given a recommendation on the amount of these products to be used daily, and the products were provided until the mothers discontinued exclusive breast-feeding (maximum 6 months). Women in the control group did not receive dietary counselling during the study visits.
Food and nutrient intakes were evaluated using a 3 d food record, including one weekend day, at the first, second and third trimesters of pregnancy and at 1 month postpartum. Daily energy and nutrient intakes were calculated using the Micro-Nutrica® software program version 2.5 (Research Centre of the Social Insurance Institution).
Clinical evaluation
Information on age, parity, smoking and education was obtained by interviews. Weight before pregnancy was taken from the records of the maternal welfare clinic and used in the calculation of pre-pregnancy BMI as weight (kg) divided by the square of height (m2). Weight during pregnancy was measured by a research nurse at every study visit and height at the first visit. Women's weight before labour was recorded at the last prenatal visit to a maternal welfare clinic or in hospital within 1 week before delivery, and used for the determination of the total weight gain during pregnancy. Weight gain during pregnancy was classified as normal, excessive or low as previously described elsewhere(22). Infants' weights at birth were obtained from hospital records; at 1 month of age it was measured by the research nurse. The prevalence of atopy in women was defined as atopic sensitisation assessed by skin prick tests at the third trimester of pregnancy, as described elsewhere(Reference Huurre, Laitinen and Rautava23).
Sampling and analytical methods
Venous blood samples were taken from women after overnight fasting at the first and third trimesters of pregnancy and at 1 month postpartum. Children's blood samples were taken at 1 month of age without fasting. Serum samples were stored in − 70°C until the analysis in the NIHR Cambridge Biomedical Research Centre, Core Biochemical Assay Laboratory.
Analyses were performed with an in-house, two-site, microtitre plate-based, time-resolved fluorometric (dissociation-enhanced lanthanide fluorescent immunoassay, DELFIA) assay (Perkin-Elmer) using DELFIA multibuffer as an assay buffer and as a diluent for biotinylated antibody and streptavidin. Briefly, the microtitre plate was coated with a monoclonal anti-leptin-capture antibody before the sample was added to the plate along with DELFIA multibuffer. After the incubation and washing of the plate, biotinylated polyclonal anti-human leptin detection antibody was added and the plate was incubated and washed again. Finally, europium-labelled streptavidin was added, the plate was washed and the enhancement solution was added to generate fluorescence. Assays were run in duplicate in 20 μl of sample per well and dilution series of human recombinant leptin were used as a calibrator. The lower limit of detection was 0·1 ng/ml (in-house data), and the between-batch imprecision was 7·1 % at 2·7 ng/ml, 3·9 % at 14·9 mg/ml and 5·7 % at 54·9 ng/ml (in-house data). Antibodies and standards were purchased from R&D Systems Europe.
Statistics
Characteristics of the women and children are presented as means with ranges or as a proportion of the study subjects. Dietary intakes of energy and nutrients are shown as the intake during pregnancy (mean of the first, second and third trimesters) and after pregnancy (mean of 1 and 6 months postpartum). ANOVA was used to study the differences between the groups in the intake of energy and energy-yielding nutrients.
Serum leptin concentrations measured in women at the first and third trimesters of pregnancy and at 1 month postpartum as well as in the cord blood and infant serum at 1 and 6 months of age were the primary variables in the present study. ANCOVA was used to compare the women's serum leptin concentrations between the groups. Baseline (measured at the first trimester of pregnancy) leptin concentration was included as a covariate. Results are given as geometric means with 95 % CI. ANOVA was used for the comparison of leptin concentrations in children's serum. Results are given as geometric means with 95 % CI. ANCOVA for repeated measures for women and ANOVA for repeated measures for children were conducted in order to study within-subject changes in serum leptin concentrations. Group effect, time effect and interactions between group and time were all analysed.
As an exploratory analysis, the associations between maternal characteristics and serum leptin concentrations were studied further. First, in order to identify potential explanatory factors for serum leptin concentrations in women, cord blood and in children, Pearson's correlations (including factors such as maternal age, pre-pregnancy weight and BMI, dietary intake of energy and nutrients, smoking before or during pregnancy and duration of lactation in women, and, additionally, sex, birth weight and weight at 1 and 6 months of age in offspring) were used. ANOVA was also used to study the differences in serum leptin concentrations according to women's BMI ( < 25 or ≥ 25 kg/m2), weight gain during pregnancy (less or within the recommendation, or more than recommended), atopy (skin prick test positivity or negativity), smoking before or during pregnancy (yes or no), exclusive breast-feeding at 1 month of age (yes or no) and breast-fed at 6 months of age (yes or no). The associations between dietary intake and leptin concentrations were studied further by dividing women into tertiles according to their intake of energy and nutrients (total fat, SFA, MUFA, PUFA, protein, carbohydrates, sucrose, fructose, glucose, total fibre, soluble fibre and non-soluble fibre) both as quantitatively and as relative to energy intake (percentage of energy). Then, ANOVA was used to study the differences in serum leptin concentrations between the tertiles. Thereafter, multivariate forward stepwise regression models were applied. The interventions (dietary counselling and probiotic supplementation) were forced to all models, and other factors introduced into the women's model were baseline leptin concentration at the beginning of pregnancy, pre-pregnancy BMI, weight gain during pregnancy, intakes of energy, protein, carbohydrates, fibre and SFA as a mean of the third trimester and 1 month postpartum as continuous variables. In the cord blood models, the included factors were dietary counselling, probiotic supplementation, maternal pre-pregnancy BMI, weight gain during pregnancy and maternal intakes of energy, protein, carbohydrates, fibre and fructose as a mean of the first, second and third trimesters of pregnancy as well as birth weight and sex of the infant. When modelling infant serum leptin concentrations, dietary counselling and probiotic supplementation were again forced into the model and other factors included were maternal pre-pregnancy BMI and maternal serum leptin concentration as a mean of the third trimester and 1 month postpartum, maternal dietary intake of energy, protein, carbohydrates and fibre as a mean of the third trimester of pregnancy and 1 month postpartum as well as sex and birth weight of the infant. The criterion for entry was P< 0·10 to achieve a model with significant or nearly significant factors combined with the effect of dietary counselling and probiotic supplementation.
All statistical analyses were performed on SPSS for Windows (version 15.0; SPSS, Inc.).
Results
Subject characteristics
Subject characteristics are shown in Table 1. All women were Caucasian, in good health and well educated; 81 % in the control group, 71 % in the dietary counselling with placebo group and 79 % in the dietary counselling with probiotics group had completed a college or university education. The dietary intakes of energy and energy-yielding nutrients are shown in Table 2.
Table 1 Characteristics of women and their infants in the study groups (Mean values, ranges and percentages)

Table 2 Dietary intake of energy and energy-yielding nutrients in women (Mean values and 95 % confidence intervals)
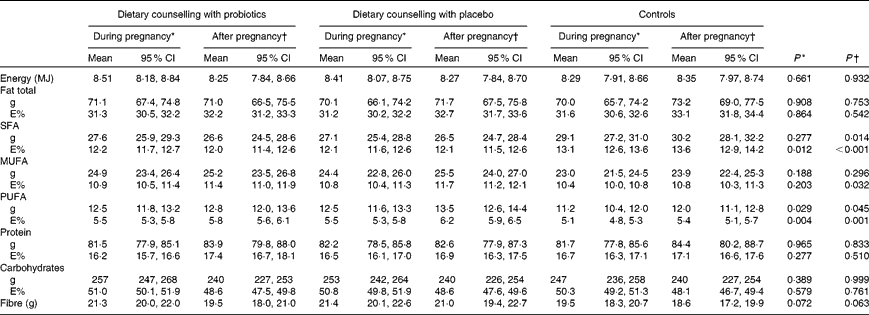
E%, percentage of energy.
* The mean of the intakes at the first, second and third trimesters of pregnancy.
† The mean of the intakes at 1 and 6 months postpartum.
Serum leptin concentrations in women
Women's serum leptin concentrations increased significantly from the first to the third trimester of pregnancy (P< 0·001), decreasing again after delivery (P< 0·001) (Table 3). Dietary counselling or probiotic supplementation had no influence on women's serum leptin concentrations (Tables 3 and 4).
Table 3 Serum leptin concentrations (ng/ml) in women and their children (Geometric mean values and 95 % confidence intervals)
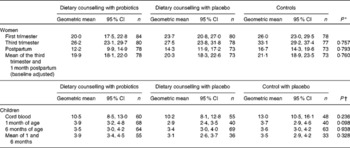
* Group comparisons were performed using ANCOVA for repeated measures, where the baseline (first-trimester) concentration was included as a covariate. The results are given as baseline-adjusted marginal means with 95 % CI.
† Group comparisons were performed using ANOVA.
Table 4 Multivariate regression models explaining the women's (mean of the third trimester and 1 month postpartum) serum leptin concentrations (Regression coefficients and 95 % confidence intervals)

Statistical analyses suggested that women's baseline leptin concentration as well as women's pre-pregnancy BMI and weight gain during pregnancy explained their serum leptin concentrations during the follow-up (Table 4). Indeed, overweight women (pre-pregnancy BMI >25 kg/m2) had significantly higher serum leptin concentration (34·77 ng/ml) when compared with normal-weight women (BMI < 25 kg/m2, 19·12 ng/ml) (P< 0·001). Furthermore, women who gained weight more than what is recommended during pregnancy had higher leptin concentrations (29·36 ng/ml) compared with women whose weight gain was within the recommendations or less than recommended (18·88 ng/ml) (P< 0·001). An inverse relationship of serum leptin concentration with dietary fibre intake and positive with the intake of SFA was detected (Table 4). The associations between dietary intake and leptin concentration were studied further by dividing women into tertiles according to their fibre and SFA (mean of the third trimester and 1 month postpartum) intakes. These analyses revealed that women in the lowest tertile of fibre intake as adjusted for energy intake had higher serum leptin (mean of the third trimester and 1 month postpartum) concentration (25·68 ng/ml) compared with women in the middle (22·55 ng/ml) or highest (19·33 ng/ml) tertile of fibre intake (P= 0·033). Instead, no significant difference in leptin concentrations between the tertiles was detected based on the women's dietary intake of SFA as quantitatively (T1: 20·76 ng/ml, T2: 23·43 ng/ml and T3: 24·03 ng/ml, P= 0·349) or as adjusted for energy intake (T1: 20·41 ng/ml, T2: 22·69 ng/ml and T3: 24·17 ng/ml, P= 0·276).
Cord blood and infant serum leptin concentrations
Leptin concentration was significantly higher in the cord blood when compared with concentrations in children's serum at 1 month of age (P< 0·001), whereas no significant change was detected between 1 and 6 months of age (P= 0·407) (Table 3). No significant differences were found in children's serum leptin concentrations according to the mother's study group (Table 3).
Based on the statistical analyses, explanatory factors for cord blood leptin concentrations were infant birth weight and sex as well as maternal leptin concentration and protein intake during pregnancy (Table 5). Indeed, the mean cord blood leptin concentration was significantly lower in boys (8·59 ng/ml) compared with girls (14·81 ng/ml) (P< 0·001). When dividing infants into tertiles according to their birth weight, cord blood leptin concentration was significantly lower in the lowest tertile of birth weight (T1, 7·32 ng/ml) compared with the middle (T2, 11·79 ng/ml) or highest (T3, 15·35 ng/ml) tertile (P>0·001). Similarly, in the lowest tertile of maternal leptin concentration, cord blood leptin concentration was significantly lower (T1, 7·90 ng/ml) compared with the middle (T2, 10·86 ng/ml) or highest (T3, 13·73 ng/ml) tertile (P= 0·002). Instead, when dividing mothers according to their protein intake during pregnancy, cord blood leptin concentration was significantly lower in the highest protein intake tertile (T3, 8·18 ng/ml) compared with the middle (T2, 13·15 ng/ml) or lowest (T1, 12·13 ng/ml) tertile of protein intake (P= 0·005).
Table 5 Multivariate forward stepwise regression model explaining cord blood leptin concentrations (Regression coefficients and 95 % confidence intervals)
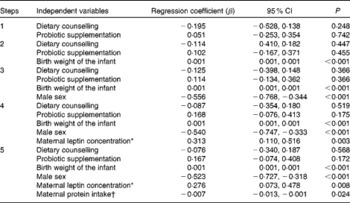
* Maternal leptin concentration taken into account as a mean of the first- and third-trimester concentrations for logarithmic conversion.
† Maternal protein intake taken into account as a mean intake during pregnancy.
Birth weight and sex determined serum leptin concentrations also during infancy (mean of 1 and 6 months of age; Table 6). Boys still had significantly lower serum leptin concentrations at 1 month of age (3·03 ng/ml) compared with girls (4·20 ng/ml) (P= 0·007), whereas at 6 months of age, no significant difference in leptin concentration between male (3·21 ng/ml) and female (3·83 ng/ml, P= 0·063) infants was detected. Maternal characteristics, leptin concentrations, dietary factors or dietary counselling of pregnant women did not significantly explain serum leptin concentrations during infancy, but according to the multivariate regression model, probiotics tended to increase infant leptin concentration (P= 0·090; Table 6).
Table 6 Multivariate regression models explaining children's (mean of 1 and 6 months of age) serum leptin concentrations (Regression coefficients and 95 % confidence intervals)
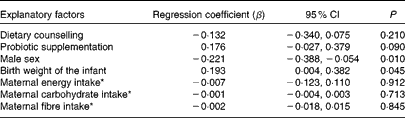
* Maternal intake of energy, carbohydrates and fibre as a mean of the third trimester and 1 month postpartum.
The positive relationship between infant weight and leptin concentration was detected in all the studied time points: at birth, r 0·457, P< 0·001; at 1 month of age, r 0·298, P< 0·001; at 6 months of age, r 0·492, P< 0·001. Additionally, a weak negative association between cord blood leptin concentration and infant weight at 6 months of age was found (r − 0·163, P= 0·044).
Discussion
In the present study, we described the increase in women's serum leptin concentrations from the first to the third trimester of pregnancy and then the decrease after delivery. The key determinant of leptin concentration in women seemed to be their weight status including both pre-pregnancy BMI and weight gain during pregnancy. The novel findings here suggest that a high dietary intake of fibre and a low intake of SFA may help to control serum leptin concentrations in women. Infants' leptin concentrations at 1 month of age were significantly decreased from the cord blood levels. Explanatory factors for cord blood leptin concentrations seemed to be the birth weight and sex of the infant as well as maternal leptin concentration and protein intake during pregnancy. During infancy, only birth weight and sex remain significant explanatory factors for leptin concentration.
We showed here that the elevation in leptin concentrations during pregnancy is in good agreement with earlier studies(Reference Henson and Castracane24). The moderate rise in leptin concentration most probably represents a normal pregnancy-related phenomenon leading to leptin resistance, which, in turn, ensures the adequate energy intake for both mother and child(Reference Grattan, Ladyman and Augustine25). Nevertheless, it was previously evidenced that overweight women face highly elevated leptin concentrations with the progress of pregnancy; this is predisposing to aberrations in normal pregnancy-related adaptations in maternal metabolism(Reference Vahamiko, Isolauri and Pesonen9) and further to pre-eclampsia and gestational diabetes(Reference Briana and Malamitsi-Puchner26). Indeed, here we showed that maternal pre-pregnancy overweight and excessive weight gain during pregnancy are the key determinants for highly elevated leptin concentrations during pregnancy. Therefore, it may be suggested that weight control both before and during pregnancy, in particular, avoidance of excessive weight gain during pregnancy, would provide the most effective approach to controlling the pregnancy-related increase in leptin concentration overemphasised in overweight individuals.
The novel findings here suggest that a higher dietary fibre intake and a lower intake of SFA may help to restrict the increase in leptin concentrations during pregnancy. In line with the present results, the amount of dietary fibre has been shown to be inversely associated with plasma leptin concentration in Japanese non-pregnant adults(Reference Kuroda, Ohta and Okufuji27). Clinical significance of fibre, in turn, has been evidenced both with healthy individuals and with metabolic syndrome patients who achieved significant improvements in metabolic syndrome risk factors with a high-fibre diet(Reference Tighe, Duthie and Vaughan14, Reference Pal, Khossousi and Binns28). Moreover, a high-fibre diet has been suggested to attenuate pregnancy-associated dyslipidaemia and reduce the risk of pre-eclampsia and gestational hyperglycaemia(Reference Qiu, Coughlin and Frederick29, Reference Zhang, Liu and Solomon30). Besides the beneficial effect of dietary fibre, the present study also suggests a potential detrimental impact of SFA in increasing leptin concentrations. In line with the present results and current dietary recommendations(Reference Becker, Lyhne and Pedersen31), recent epidemiological studies and randomised trials have suggested that replacing saturated fat with polyunsaturated fat decreases the risk of CHD(Reference Siri-Tarino, Sun and Hu32). Furthermore, during pregnancy, a lower dietary intake of SFA has been proposed for decreasing the risk of gestational hyperglycaemia(Reference Bo, Menato and Lezo33). Altogether, these findings suggest that a diet rich in fibre and low in saturated fat may provide both short- and long-term health benefits to both women and their children by controlling the pregnancy-related increase in leptin concentration.
Similar to earlier studies(Reference Trevino-Garza, Bosques-Padilla and Estrada-Zuniga34, Reference Mellati, Mazloomzadeh and Anjomshoaa35), the present results suggest here that both cord blood serum and infant serum leptin concentrations are positively associated with infants' birth weight and female sex. The difference in leptin concentrations between the sexes may result from a varying amount and distribution of body fat in infants(Reference Trevino-Garza, Bosques-Padilla and Estrada-Zuniga34). On the other hand, sex-specific adaptations taking place during the fetal period in the placenta may explain the difference in leptin concentrations. Indeed, these adaptations have been suggested, in turn leading to differences in growth and metabolic and immune functions between female and male infants(Reference Clifton36). Interestingly, higher maternal dietary protein intake during pregnancy seemed to decrease cord blood leptin concentration. Nevertheless, the clinical significance of this finding remains to be shown.
Breast milk leptin concentrations were not measured in the present study. We acknowledge that it is a weakness of the study since others have suggested that breast milk leptin could provide a link between maternal body composition and infant growth(Reference Schuster, Hechler and Gebauer37, Reference Miralles, Sanchez and Palou38). Here we found a positive relationship between infant weight and leptin concentration in all the studied time points and, additionally, a weak negative correlation between cord blood leptin concentration and infant weight at 6 months of age. This is in line with earlier studies suggesting that lower cord blood leptin levels are associated with pronounced weight gain(Reference Mantzoros, Rifas-Shiman and Williams39) and that higher neonatal leptin predicts slower weight gain(Reference Parker, Rifas-Shiman and Belfort40) in the first 6 months of life. Nevertheless, no conclusions about the relationships between early-life leptin levels and later obesity risk can be drawn based on the present results, but still the present study with a unique study population comprises an encouraging starting point for future analysis.
In summary, we conclude that particularly in overweight women, weight control both before and during pregnancy as well as the modification of diet towards higher fibre and lower SFA intakes may provide an effective approach to controlling the pregnancy-related increase in leptin concentration in women. This may consequently lead to more balanced maternal metabolism during pregnancy and provide later health benefits for both women and their children.
Acknowledgements
We would like to thank Ms Ulla-Maija Eriksson for the clinical work she conducted with the study subjects and Ms Satu Leinonen for the technical work. The present study was supported by grants from the Social Insurance Institution of Finland, the Sigrid Juselius Foundation, the Jenny and Antti Wihuri Foundation (personal S. V.) and the Juho Vainio Foundation (personal S. V.). The food products were provided by Raisio plc (Raisio). The authors' responsibilities were as follows: K. L. and E. I. contributed to the study concept and design; S. V. and K. L. carried out the collection of the data. All authors participated in the preparation of the manuscript. None of the authors had a conflict of interest to declare.








