Individuals who undertake high levels of physical activity maintain energy balance and stable body weights more effectively than their sedentary peers(Reference Wareham, van Sluijs and Ekelund1, Reference Saris, Blair and van Baak2) and long-term maintenance of weight loss in the formerly obese is facilitated by high physical activity(Reference Jeffery, Wing and Sherwood3, Reference Schoeller4). Thus, over the long term, the interaction between energy intake and energy expenditure for the regulation of energy balance appears to differ between high energy turnover (i.e. high physical activity) and low energy turnover (i.e. low physical activity) conditions. As maintenance of energy balance and body weight over the long term is the culmination of repeated short-term interactions between energy expenditure and energy intake, short-term metabolic studies can help understanding of the physiological and behavioural mechanisms involved in the regulation of energy balance under high or low energy turnover conditions.
A number of reports have evaluated the effects of an exercise session on subsequent energy metabolism, reporting post-exercise increases in fat oxidation immediately(Reference Votruba, Atkinson and Hirvonen5, Reference Votruba, Atkinson and Schoeller6) and for up to 24 h(Reference Hansen, Shriver and Schoeller7, Reference Gill, Frayn and Wootton8), although this is not unequivocal(Reference Melanson, MacLean and Hill9). This shift in metabolism towards fat oxidation may be important for body-weight regulation, as high levels of fat oxidation, measured either at rest in the fasted state(Reference Seidell, Muller and Sorkin10, Reference Marra, Scalfi and Covino11) or over 24 h(Reference Zurlo, Lillioja and Esposito-Del Puente12) have been shown to be protective against subsequent long-term weight gain, independent of metabolic rate. The acute effects of an exercise session on subsequent appetite regulation and feeding behaviour have also been investigated. Studies measuring food intake have fairly consistently shown that energy intake remains unchanged immediately following an exercise session(Reference George and Morganstein13–Reference King, Lluch and Stubbs16) although relative energy intake – i.e. energy intake after accounting for the additional energy expended during exercise – is generally lower(Reference Imbeault, Saint-Pierre and Almeras15, Reference Martins, Morgan and Bloom17). Over more prolonged periods of time, evidence suggests that some degree of dietary compensation for the exercise-induced energy expenditure may occur, at least in women(Reference Stubbs, Sepp and Hughes18). Exercise may also alter macronutrient preferences, although again the literature is equivocal with some showing fat(Reference Pomerleau, Imbeault and Parker19) and others carbohydrate(Reference Westerterp-Plantenga, Verwegen and Ijedema20, Reference Stubbs, Hughes and Johnstone21) to be preferentially consumed after exercise, whilst others observe no difference in macronutrient consumption(Reference Hubert, King and Blundell14, Reference King, Lluch and Stubbs16). Exercise-induced changes in appetite and feeding behaviour may, in part, be explained by fluctuations in appetite hormones, such as ghrelin. Although studies have consistently shown no change in total ghrelin in response to exercise(Reference Martins, Morgan and Bloom17, Reference Burns, Broom and Miyashita22–Reference Kraemer, Durand and Acevedo24), a reduction in acylated ghrelin has been observed(Reference Broom, Stensel and Bishop25–Reference Marzullo, Salvadori and Brunani28). However, the implications of this for appetite regulation and subsequent energy intake are not clear and require further investigation.
In the acute studies described above, the energy expended during the exercise session was not replaced post-exercise, making it difficult to disentangle the separate effects of exercise v. its associated energy deficit on metabolism, appetite regulation and feeding behaviour. It, therefore, remains unclear how exercise might affect metabolism and appetite when the associated energy deficit is replaced and energy balance maintained, a state that is representative of regular exercisers who maintain a stable body weight. Improved sensitivity of appetite regulation and the enhanced ability to adjust energy intake in response to prior foods eaten has been reported in those who are regularly physically active(Reference Martins, Truby and Morgan29, Reference Long, Hart and Morgan30); however, it is currently unclear whether this increased sensitivity of appetite occurs acutely in response to recent exercise. We have previously shown that fasting and postprandial fat oxidation is higher on the day following an exercise session without an associated energy deficit (high energy turnover energy balance) than following a day when no exercise was performed (low energy turnover energy balance)(Reference Burton, Malkova and Caslake31), but effects on appetite and feeding behaviour are not known, nor are the effects on metabolism during the several hours immediately post-exercise. The aim of the present study, therefore, is to determine whether differences exist in energy metabolism, appetite and feeding behaviour over the course of a day between high energy turnover and low energy turnover energy balance conditions.
Methods
Subjects
A total of thirteen premenopausal women were recruited to the present study. Their physical characteristics were age 32 (sd 8) years, BMI 27·5 (sd 1·1) kg/m2, waist circumference 87·1 (sd 6·2) cm and maximal oxygen uptake (VO2max) 34·7 (sd 5·8) ml/kg per min. All of the women were apparently healthy non-smokers, with no known history of CVD or diabetes. None was taking any medication thought to interfere with lipid or energy substrate metabolism. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Faculty of Biomedical and Life Sciences Ethics Committee at the University of Glasgow (Glasgow, UK). Each subject gave written informed consent before participation.
Experimental design
After preliminary testing, each subject completed two separate 1 d trials: energy balance with low energy turnover (low energy turnover) and energy balance with high energy turnover (high energy turnover). Trials were performed in a randomised design and completed at an interval of 4 weeks to avoid potential confounding effects of the menstrual cycle. An overview of the study protocol is shown in Fig. 1. For 3 d before each experimental trial, subjects were instructed to avoid alcohol and all exercise, other than activities of daily living. During the 3 d preceding their first trial, subjects recorded all of the food and drink that they consumed and were instructed to replicate this diet for the 3 d preceding their second experimental trial.
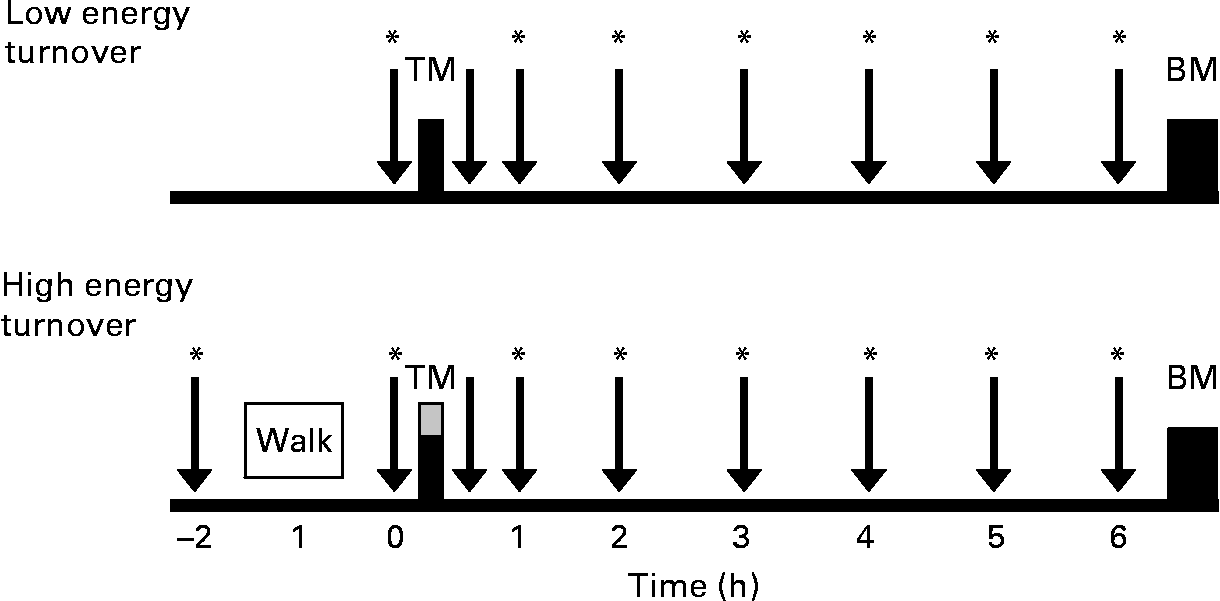
Fig. 1 Study design. Subjects completed two separate trials: low energy turnover and high energy turnover. In the high energy turnover trial, the net energy expenditure of exercise was replaced (![]() ) at the test meal (TM). Regular blood samples and appetite questionnaires ( ↓ ) and expired air measurements (*) were collected. The buffet meal (BM) was completed 6·5 h after the test meal.
) at the test meal (TM). Regular blood samples and appetite questionnaires ( ↓ ) and expired air measurements (*) were collected. The buffet meal (BM) was completed 6·5 h after the test meal.
Preliminary sessions
A four-stage preliminary submaximal treadmill test(Reference Balady, Berra and Golding32) was completed before the first experimental trial to estimate VO2max and to calculate the speed and gradient that was required to elicit an intensity of 50 % VO2max, the intensity that was used for the exercise in the high energy turnover trial.
Before the first experimental trial, subjects attended the metabolic suite having fasted overnight for at least 12 h for measurement of RMR. For this, and both experimental trials, subjects travelled to the laboratory using motorised transport to minimise physical activity before arrival. After 20 min of rest lying supine on a couch, metabolic rate was determined by indirect calorimetry using a ventilated hood (Deltatrac Metabolic Monitor; Datex Engstrom, Sidcup, Kent, UK). Expired air measurements were collected over 25 min and the mean value for the final 20 min was used to calculate RMR.
During the preliminary phase, individual subjects were also asked to complete a questionnaire providing details of: (i) foods they did not like; (ii) specific dietary requirements, for example, vegetarian; and (iii) all food allergies. This information was used to ensure that foods provided in the ad libitum buffet meal were suitable for subjects to consume.
Low energy turnover trial
Subjects attended the metabolic suite at 10.00 hours having fasted from 20.00 hours the evening before. Following a 10 min rest lying on a couch, a 25 min fasting expired air sample was collected (Deltatrac Metabolic Monitor; Datex Engstrom). A cannula was introduced into an antecubital vein, and after a 10 min interval, a fasting blood sample was taken (0 h on Fig. 1), on completion of which a 10 cm visual analogue scale (VAS) questionnaire was used to rate their hunger, satiety, fullness, prospective food consumption and desire to eat(Reference Flint, Raben and Blundell33).
Once fasting measurements were made, the test breakfast was provided, the energy content of which was based on their expected energy expenditure during the 6 h observation period and designed to maintain energy balance. Energy content was individually calculated using RMR (in kJ/h) × 6 × 1·2, where 1·2 represents an activity level of an individual who is chair or bed bound(Reference Shetty34). The meal comprised a bagel, polyunsaturated fat margarine and a meal-replacement drink (Complan; Complan Foods Ltd, London, UK) made with whole milk, and in total provided 49 % of energy as carbohydrate, 37 % as fat and 14 % as protein. On completion of the meal, subjects underwent a 6 h postprandial observation period, during which regular blood samples and VAS appetite questionnaires were completed at 0·5, 1, 2, 3, 4, 5 and 6 h after the test meal. Expired air measurements were made during the 15 min preceding the 1, 2, 3, 4, 5 and 6 h blood samples. At 30 min after the final blood sample was collected (6·5 h after the test meal), subjects were provided with a buffet meal, which is described below. During both the test meal and buffet meal, subjects completed VAS food palatability questionnaires rating the meals' appeal, smell, taste, aftertaste and palatability. Water was provided ad libitum during the postprandial period in the first trial with the volume and pattern repeated during the subsequent trial.
High energy turnover trial
Subjects attended the metabolic suite at 08.00 hours having fasted from 20.00 hours the previous evening. Fasting measurements were made ( − 2 h on Fig. 1) as described for the low energy turnover trial before subjects completed a 60 min treadmill walk at 50 % VO2max. At rest before the walk, at 15 min intervals during the walk and for 10 min after completion of the walk expired air samples were collected in Douglas bags for the determination of oxygen uptake and carbon dioxide production. Heart rate and rate of perceived exertion(Reference Borg35) were recorded at 15 min intervals during the walk. Water was provided ad libitum during and after the walk. On completion of the walk, subjects were allowed to shower and change before fasting post-exercise (0 h on Fig. 1) expired air and blood samples and appetite questionnaires were repeated. Subjects were then provided with a test meal with the same composition as that consumed in the low energy turnover trial, but the energy content provided was RMR (in kJ/h) × 6 × 1·2 plus 107 % of the net energy expenditure of the treadmill walk, to ensure that energy balance status post-breakfast was the same as in the low energy turnover trial. We replaced 107 %, rather than 100 %, of the energy during exercise at breakfast to account for the elevated metabolic rate during recovery from exercise which is typically in the order of 2–7 % of the exercise-induced energy expenditure(Reference Gore and Withers36–Reference Sedlock, Fissinger and Melby38). After the test meal, a 6 h postprandial observation period and buffet meal were completed as described for the low energy turnover trial and food palatability questionnaires were collected following both meals.
Ad libitum buffet meal
Exactly the same buffet meal was provided in both trials with a variety of foods offered, including cheese and tomato pasta bake, salad and salad dressing, bread, butter, fruit cocktail, chocolate cake and cream. Overall, 46 % of the energy in the foods provided was carbohydrate, 44 % was fat and 10 % was protein. All foods were individually weighed and presented to the subject in quantities in excess of expected consumption and in a way that avoided any effect of portion size on typical feeding behaviour. Meals were consumed in isolation to avoid the impact of other people on food consumption(Reference Herman and Polivy39). Subjects were asked to eat until they reached their normal degree of satiation and 30 min was allowed for the meal, during which time no water was allowed. The weight of food consumed was calculated by subtracting the amount of food left from the original amount provided, the energy and nutrient content of which was determined using the food manufacturers' labels and a dietary analysis program (CompEat version 5; Nutrition Systems, Grantham, Lincs, UK). Subjects were blinded to the purpose of the buffet meal (i.e. measuring food intake), and were instead advised that the aim was to investigate the effects of exercise on markers of food palatability. This was done to reduce the potential bias that can occur if a person is consciously aware that food consumption is being monitored, as eating patterns and behaviour can be affected by a number of emotional and cognitive beliefs and restraints, especially with regards to the volume of food that is consumed and types of food that are perceived ‘good or bad’(Reference Herman and Polivy39).
Blood analysis
Blood samples were collected into K-EDTA tubes and placed on ice. All samples were separated within 15 min of collection and stored at − 80°C until analysis. Before centrifugation, 1·98 ml blood was extracted and treated with p-hydroxymercuribenzoic, centrifuged, and added to 1 m-hydrochloric acid solution in preparation for acylated ghrelin analysis. Plasma TAG, glucose and NEFA concentrations were determined by enzymic colourimetric methods using commercially available kits (Roche Diagnostics GmbH, Mannheim, Germany and Randox Laboratories Ltd, Crumlin, Co. Antrim, UK). Insulin and acylated ghrelin were determined using commercially available ELISA (Mercodia AB, Upsala, Sweden; Phoenix Europe GmbH, Karlsruhe, Germany and SPI-BIO, Montigny Le Bretonneux, France). All samples for each subject were analysed in a single analyser run. CV were < 3·1 % for all non-ELISA assays, 3·9 % for the insulin ELISA and 5·9 % for the acylated ghrelin ELISA.
Statistical analysis
Energy expenditure and energy substrate utilisation were calculated using indirect calorimetry(Reference Frayn40). For these calculations urinary N excretion was assumed to be 0·11 mg/kg per min throughout each trial, based on data from previous studies in the literature(Reference Flatt, Ravussin and Acheson41, Reference Melanson, Donahoo and Dong42). We consider this assumption to be justified, as data from previous studies have demonstrated that exercise with similar energy expenditures to the exercise intervention used in the present study, either with or without an energy deficit, does not influence postprandial protein oxidation rates(Reference Votruba, Atkinson and Hirvonen5, Reference Herman and Polivy39, Reference Melanson, Sharp and Seagle43). The total area under the 6 h variable v. time curves (AUC), divided by the duration of the observation period (6 h), i.e. the time-averaged AUC, were used as summary measures of the postprandial responses. Cumulative energy and energy substrate balances were calculated by summing the AUC for individual time periods throughout each experimental trial.
Data were analysed using Statistica (version 6.0; StatSoft Inc., Tulsa, OK, USA) and Minitab (version 13.1; Minitab Inc., State College, PA, USA). Before any statistical analysis, all data were tested for normality using the Anderson–Darling normality test. Only acylated ghrelin data were found not to display a normal distribution and thus these values were normalised by logarithmic transformation (base 10) before any further statistical analysis (prior units pg/ml). Differences between trials for measurements made in the fasted state and summary postprandial responses were compared using paired-sample t tests with differences over time for both trials calculated using two-way ANOVA with repeated measures for trial and time. Post hoc Tukey tests were used to identify where differences lay. Relationships between variables were assessed using Pearson product-moment correlations. Statistical significance was accepted at the P < 0·05 level and data are presented as mean values with their standard errors unless otherwise stated.
Results
Responses during treadmill walk
Subjects walked for 1 h at a speed of 5·5 (sem 0·1) km/h up a gradient of 2·4 (sem 0·6) %. All subjects completed the walk without difficulty, rating the exercise as ‘light’ (11·1 (sem 0·3)) on the Borg scale of 6–20(Reference Borg35). Mean VO2 during the walk was 16·6 (sem 0·7) ml/kg per min (equivalent to 47 % VO2max) and mean heart rate was 123 (sem 2) beats/min. Net energy expenditure of the walk was 1·18 (sem 0·07) MJ, and there was an additional net energy expenditure of 0·07 (sem 0·02) MJ during recovery following exercise before energy expenditure returned to baseline. Net fat and carbohydrate oxidation during the walk was 22·3 (sem 1·4) g and 18·1 (sem 3·3) g, respectively.
Energy and substrate utilisation in the fasted state
In the low energy turnover trial, a fasting expired air sample was collected immediately before breakfast (0 h), and in the high energy turnover trial fasting expired air samples were collected pre-exercise ( − 2 h) and post-exercise (0 h). Fasting energy expenditure was not different between trials (low energy turnover, 251·3 (sem 6·5) kJ/h; high energy turnover pre-exercise, 251·5 (sem 6·3) kJ/h; P = 0·976) or within the high energy turnover trial itself (high energy turnover post-exercise, 253·5 (sem 6·6) kJ/h; P = 0·583) and there was also no difference between low energy turnover fasting and high energy turnover pre-exercise fat oxidation rates (low energy turnover, 3·73 (sem 0·37) g/h; high energy turnover pre-exercise, 3·45 (sem 0·32) g/h; P = 0·407). Post-exercise fat oxidation (4·25 (sem 0·26) g/h) was, however, significantly higher than pre-exercise in the high energy turnover trial (P = 0·007). Post-exercise carbohydrate oxidation was lower, compared with pre-exercise, in the high energy turnover trial (post-exercise, 2·09 (sem 0·65) g/h; pre-exercise, 3·79 (sem 0·68) g/h; P = 0·023) but there was no difference in low energy turnover fasting carbohydrate oxidation (3·12 (sem 0·68) g/h) compared with either high energy turnover pre-exercise (P = 0·386) or post-exercise (P = 0·176) values.
Energy and substrate balances
Energy intake at breakfast was 1·94 (sem 0·07) MJ in the low energy turnover trial and 3·20 (sem 0·16) MJ in the high energy turnover trial. Total energy expenditure during the 6 h postprandial observation period was slightly higher in the high energy turnover trial (1·55 (sem 0·05) MJ) than the low energy turnover trial (1·47 (sem 0·04) MJ; P = 0·008). This small difference is likely to reflect a greater thermic effect of food in the high energy turnover trial due to the larger energy intake at breakfast. However, there were no significant differences between trials in fat (low energy turnover, 18·7 (sem 1·6) g; high energy turnover, 20·1 (sem 1·5) g; P = 0·350) or carbohydrate (low energy turnover, 26·5 (sem 0·35) g; high energy turnover, 28·9 (sem 3·5) g; P = 0·480) oxidation over this period.
Cumulative energy, fat and carbohydrate balances during each trial are shown in Fig. 2. By design, cumulative energy balance did not differ between trials over the course of the day, before the buffet meal. Immediately before the buffet meal, energy balance (relative to the start of the trial) was − 0·16 (sem 0·05) MJ in the low energy turnover trial and − 0·18 (sem 0·04) MJ in the high energy turnover trial (P = 0·50). However, in the low energy turnover trial this was achieved by expending 2·10 (sem 0·05) MJ of energy over the entire observation period (i.e. from − 2 h to 6 h on Fig. 1) and consuming 1·94 (sem 0·07) MJ, whereas in the high energy turnover trial, this was achieved by expending 3·39 (sem 0·12) MJ and consuming 3·20 (sem 0·16) MJ. Thus, in the high energy turnover trial, energy balance was the same as in the low energy turnover trial, but energy turnover was about 60 % greater. Energy intake in the buffet meal tended to be lower (by about 7 %) in the high energy turnover trial, although this did not achieve statistical significance (low energy turnover, 3·77 (sem 0·16) MJ; high energy turnover, 3·51 (sem 0·16) MJ; P = 0·103). However, on completion of the buffet meal cumulative energy balance was significantly lower (by about 8 %) in the high energy turnover trial (3·41 (sem 0·16) v. 3·13 (sem 0·15) MJ for the low and high energy turnover trials, respectively; P < 0·001).

Fig. 2 Cumulative energy balance (a), cumulative fat balance (b) and cumulative carbohydrate (CHO) balance (c) during the low energy turnover (○) and high energy turnover (●) trials. Times at which the test meal (□) and buffet meal (■) were provided are shown. Values are means, with standard errors represented by vertical bars. * Mean values were significantly different between trials (P < 0·001).
Postprandial cumulative fat balance was lower throughout the day in the high energy turnover trial (P < 0·05 at all time points) and remained lower after the buffet meal (low energy turnover, 17·7 (sem 3·1) g; high energy turnover 6·1 (sem 2·7) g; P < 0·001), although fat intake in the buffet meal did not differ between trials (low energy turnover, 31·0 (sem 1·9) g; high energy turnover, 29·1 (sem 1·3) g; P = 0·210). Postprandial carbohydrate balance was higher over the course of the day in the high energy turnover trial before the buffet meal (P < 0·05 at all time points), but carbohydrate intake in the buffet tended to be lower (by about 8 %) in the high energy turnover trial (low energy turnover, 128·8 (sem 5·9) g; high energy turnover, 118·7 (sem 6·7) g; P = 0·082), resulting in no difference in cumulative carbohydrate balance between trials after the buffet was completed (low energy turnover, 151·8 (sem 7·2) g; high energy turnover, 152·5 (sem 4·4) g; P = 1·000). Protein intake at the buffet meal did not differ significantly between trials (low energy turnover, 29·2 (sem 1·3) g; high energy turnover, 27·4 (sem 1·9) g; P = 0·204).
Acylated ghrelin concentrations
Due to technical problems, acylated ghrelin concentrations were not measured in two subjects; thus data are reported for eleven subjects. Log acylated ghrelin concentrations measured in the fasted state, 1 h following the walk, in the high energy turnover trial were significantly lower than values measured in the fasted state in the low energy turnover trial (low energy turnover, 1·94 (sem 0·07); high energy turnover, 1·83 (sem 0·07), P = 0·01; median values before transformation: low energy turnover, 92·6 pg/ml; high energy turnover, 65·7 pg/ml). In addition, the postprandial log acylated ghrelin response was significantly lower in the high energy turnover trial than in the low energy turnover trial (low energy turnover, 1·90 (sem 0·06); high energy turnover trial, 1·80 (sem 0·06), P = 0·04; median values before transformation: low energy turnover, 82·7 pg/ml; high energy turnover, 65·2 pg/ml) (Fig. 3).
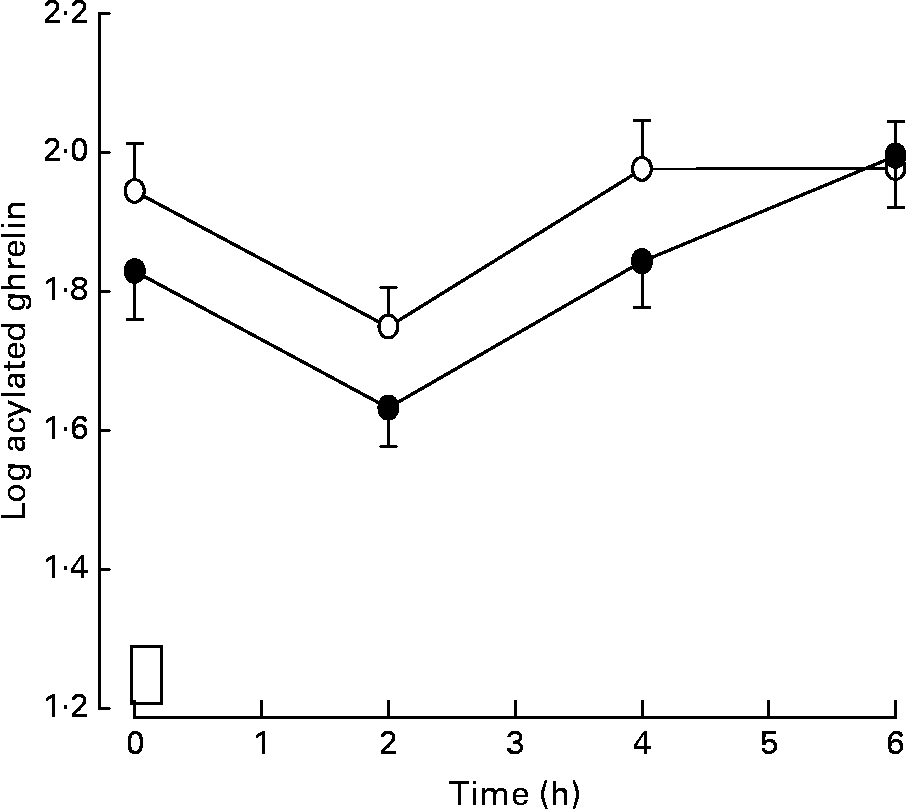
Fig. 3 Plasma log acylated ghrelin response during the low energy turnover (○) and high energy turnover (●) trials. The time at which the test meal (□) was provided is shown. Values are means, with standard errors represented by vertical bars.
Plasma metabolic variables
In the low energy turnover trial, a single blood sample, immediately before breakfast (0 h), was taken in the fasted state, and in the high energy turnover trial blood samples were taken pre-exercise ( − 2 h) and post-exercise (0 h) in the fasted state. Data from the analysis of these samples are presented in Table 1. There were no significant differences between the pre-exercise value in the high energy turnover trial and the fasting value in the low energy turnover trial for any of these variables except for TAG concentrations, which were 17 % higher in the high compared with the low energy turnover trial (P = 0·048). Compared with fasting values in the low energy turnover trial, post-exercise NEFA concentrations in high energy turnover were 34 % higher (P = 0·011). Comparisons within the high energy turnover trial itself revealed post-exercise TAG concentrations 13 % lower than pre-exercise values (P < 0·001) whilst NEFA concentrations were 36 % higher (P = 0·002). Glucose concentrations did not change post-exercise; however, insulin concentrations were 25 % lower post-exercise compared with pre-exercise (P = 0·019).
Table 1 Plasma metabolic variables in the fasted state (n 13)
(Mean values with their standard errors)
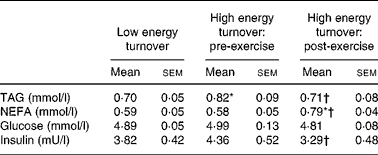
* Mean value was significantly different from that in the low energy turnover condition (P < 0·05).
† Mean value was significantly different from that pre-exercise (high energy turnover) (P < 0·05).
Summary measures of postprandial responses are given in Table 2 and the postprandial responses for TAG, NEFA, glucose and insulin are shown in Fig. 4. The postprandial NEFA response was 14 % lower (P = 0·009) and postprandial glucose and insulin responses were 7 % (P = 0·023) and 62 % (P = 0·002) higher, respectively, in the high energy turnover trial compared with the low energy turnover trial. There was no significant difference in the postprandial TAG response between trials.
Table 2 Time-averaged postprandial area under the curve values for plasma metabolic variables (n 13)
(Mean values with their standard errors)

Fig. 4 Postprandial plasma TAG (a), NEFA (b), glucose (c) and insulin (d) responses during the low energy turnover (○) and high energy turnover (●) trials. The time at which the test meal (□) was provided is shown. Values are means, with standard errors represented by vertical bars.
Subjective ratings of appetite
There were no significant differences in subjective ratings of appetite over the course of the postprandial observation period between the two trials. Time-averaged postprandial appetite ratings are shown in Table 3.
Table 3 Time-averaged postprandial area under the curve values for subjective ratings of appetite (n 13)
(Mean values with their standard errors)
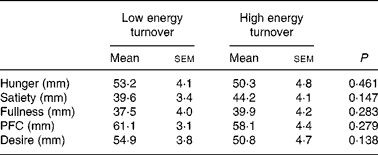
PFC, prospective food consumption.
Relationships between variables
Energy and carbohydrate intakes at the buffet meal correlated positively with total carbohydrate oxidation over the postprandial observation period (energy intake v. carbohydrate oxidation: r 0·42, P = 0·03; carbohydrate intake v. carbohydrate oxidation: r 0·52, P = 0·007) and negatively with total fat oxidation over the postprandial period (energy intake v. fat oxidation: r − 0·39, P = 0·049; carbohydrate intake v. fat oxidation: r − 0·42, P = 0·032). There was a significant negative correlation between energy intake at the meal and carbohydrate balance measured just before the buffet meal (r − 0·49; P = 0·01; Fig. 5) and a reciprocal positive correlation between energy intake and fat balance measured just before the meal (r 0·48; P = 0·01). However, energy intake was not associated with energy balance immediately before the meal (r − 0·17; P = 0·41). Furthermore, carbohydrate intake was negatively associated with carbohydrate balance before the buffet meal (r − 0·55; P = 0·004), whilst there was no relationship observed between fat balance and fat intake (r 0·16; P = 0·43). Carbohydrate balance just before the buffet meal correlated significantly positively with the plasma glucose response (r 0·49; P = 0·01) and negatively with the log acylated ghrelin response (r − 0·48; P = 0·02) at the same time point (Fig. 5). In addition, plasma glucose responses correlated negatively with the log acylated ghrelin response (r − 0·48; P = 0·02; Fig. 5). There was a tendency for a negative correlation between glucose response and energy intake at the buffet meal which did not quite achieve statistical significance (r − 0·36; P = 0·068).

Fig. 5 Scattergrams indicating the relationship between cumulative carbohydrate (CHO) balance and energy intake (r − 0·49; P = 0·01) (a), cumulative CHO balance and postprandial glucose responses (r 0·49; P = 0·01) (b), cumulative CHO balance and log acylated ghrelin responses (r − 0·48; P = 0·02) (c) and postprandial glucose responses and log acylated ghrelin responses (r − 0·48; P = 0·02) (d) in the low energy turnover (○) and high energy turnover (●) trials.
Discussion
The main finding of the present study is that high turnover energy balance influences metabolism differently from low energy turnover energy balance. Although energy balance status between these two conditions was identical over the 6·5 h postprandial observation period, fat balance was significantly lower and, reciprocally, carbohydrate balance was higher throughout the high energy turnover trial compared with the low energy turnover trial. On completion of an ad libitum buffet meal at the end of the day, energy balance was about 280 kJ more negative in the high energy turnover trial and energy intake at the buffet meal correlated strongly with carbohydrate balance immediately before the meal. In addition, circulating concentrations of acylated ghrelin – which stimulates appetite – were significantly lower over the course of the high energy turnover trial, and acylated ghrelin concentrations correlated negatively with carbohydrate balance. Thus, the present data imply that when exercise is performed and the energy expended is immediately replaced, carbohydrate balance is more positive than if exercise was not performed and that this is associated with reduced energy intake at the following meal.
The present findings – i.e. that high energy turnover energy balance led to a more positive carbohydrate balance – could conceivably have implications for the long-term regulation of body weight. Pannacciulli et al. (Reference Pannacciulli, Salbe and Ortega44) reported that 24 h carbohydrate balance, measured in a respiratory chamber, was inversely associated with daily energy intake and weight gain during a subsequent 3 d follow-up period in a cross-sectional study of 112 men and women. Furthermore, over the longer term, Eckel et al. reported that individuals who achieved more positive carbohydrate balances when consuming a high-carbohydrate diet whilst inactive gained less weight and less fat over the subsequent 4 years, suggesting that carbohydrate balance plays a strong metabolic role in long-term weight maintenance(Reference Eckel, Hernandez and Bell45). Thus, taking the present and previously reported findings(Reference Pannacciulli, Salbe and Ortega44, Reference Eckel, Hernandez and Bell45) together, it is not unreasonable to hypothesise that the enhanced ability of regular exercisers to maintain a healthy body weight may be due, in part, to the more positive carbohydrate balance induced by high energy turnover, leading to a reduction in relative energy intake. It is, however, important to exert a note of caution at this point. The present study was short term and did not measure participants' energy intakes after they left the laboratory, so it is not known whether or not compensatory changes to energy intake might occur over a longer time-frame. Thus, further longer-term studies are needed to confirm the present observations before firm conclusions can be reached about the role of exercise-induced alterations in carbohydrate balance in long-term weight maintenance.
The more positive carbohydrate balance in the high energy turnover trial implies higher muscle and/or liver glycogen stores. It is well established that muscle glycogen and liver resynthesis is enhanced in the hours immediately post-exercise(Reference Casey, Mann and Banister46, Reference Price, Rothman and Taylor47) and that during this time there is increased partitioning of ingested carbohydrate towards glycogen resynthesis rather than oxidation(Reference Kiens and Richter48). Thus, despite carbohydrate intake at breakfast being about 65 % higher in the high energy turnover trial than the low energy turnover trial (about 96 g v. about 58 g of carbohydrate ingested), and higher postprandial glucose concentrations in the high energy turnover trial, carbohydrate oxidation over the postprandial observation period did not differ between trials. This is in contrast to situations when carbohydrate intake is increased in the absence of prior exercise, which results in a marked increase in carbohydrate oxidation(Reference Acheson, Schutz and Bessard49).
One interesting observation in the present study is that ad libitum food consumption at the buffet meal led to identical levels of carbohydrate balance in the low and high energy turnover trials on completion of the meal, suggesting that individuals ate until a ‘set point’ for carbohydrate balance was achieved. This fits well with observations of Flatt who, more than 20 years ago, reported that, in mice, energy intake was strongly related to the previous day's carbohydrate balance and proposed that, in contrast to fat stores, carbohydrate stores in the body were tightly regulated(Reference Flatt50). More recent studies manipulating glycogen stores in human subjects have had mixed results. Snitker et al. found that in normal-weight young white men ad libitum energy intake was inversely related to carbohydrate balance on the previous day, with carbohydrate balance explaining 9 % of the variance in energy intake(Reference Snitker, Larson and Tataranni51). In contrast, carbohydrate balance explained about 24 % of the variance in energy intake and 30 % of the variance in carbohydrate intake in the present study. However, in the earlier report ad libitum food intake was approximately twice as high as energy expenditure (>20 MJ/d and about 10 MJ/d)(Reference Snitker, Larson and Tataranni51), suggesting that other external factors, such as availability of large quantities of highly palatable food, may have led to hedonic factors over-riding endogenous homeostatic appetite signals(Reference Finlayson, King and Blundell52). Shetty et al. reported that manipulating carbohydrate balance by altering the carbohydrate content of the diet did not influence energy intake, but in the present study the achieved differences in carbohydrate balances between diets were only about a third of the difference in carbohydrate intake due to autoregulatory changes to carbohydrate oxidation(Reference Shetty, Prentice and Goldberg53). In contrast, the increased carbohydrate intake in the high energy turnover trial in the present study did not lead to increased carbohydrate oxidation. Thus, the scenario of high v. low energy turnover appears to influence substrate metabolism and subsequent energy intake differently from the manipulation of carbohydrate balance by altering the composition of isoenergetic diets.
Studies which have investigated the effects of an exercise session on circulating acylated ghrelin in adults have reported decreases in concentration of the hormone during and immediately following exercise(Reference Broom, Stensel and Bishop25, Reference Broom, Batterham and King27, Reference Marzullo, Salvadori and Brunani28) but the effects on acylated ghrelin concentrations during the hours following an exercise session are less clear(Reference Broom, Stensel and Bishop25, Reference Broom, Batterham and King27). The present data demonstrate that when energy intake is increased to maintain energy balance, the exercise-induced attenuation in acylated ghrelin concentrations persists for at least 6 h. Interestingly, however, this occurred without any difference in subjective ratings of appetite between trials although the ghrelin response was associated significantly with carbohydrate balance, which, in turn, was strongly associated with energy intake. While it is possible that the VAS questionnaires used in the present study had insufficient power to detect subtle differences in appetite sensations, the present data may indicate that any potential role of acylated ghrelin in the regulation of feeding behaviour was not occurring at an overtly conscious level. Furthermore, the finding that questionnaires could not detect differences in sensations of appetite between the two trials, despite clear differences in energy balance being evident after the buffet meal, highlights the importance of using direct measures of food consumption, and not relying on reported sensations of appetite, in studies attempting to elucidate the effects of exercise on energy balance and feeding behaviour.
Although not definitive on this issue, our data may help elucidate the ‘signal’ which relates carbohydrate balance to appetite. Glucose and acylated ghrelin responses both correlated significantly with carbohydrate balance, and with each other, and glucose concentrations were significantly higher, and acylated ghrelin concentrations significantly lower, in the high energy turnover trial than the low energy turnover trial. Evidence from the literature suggests that it is likely that blood glucose concentration plays a role in the regulation of appetite. Spontaneous meal ingestion synchronises well with transient declines in blood glucose concentration(Reference Melanson, Westerterp-Plantenga and Campfield54), and glucose infusion has been shown to suppress food intake(Reference Chapman, Goble and Wittert55) and increase satiety(Reference Gielkens, Verkijk and Lam56). In addition, it has been reported that energy intake at an ad libitum meal was significantly negatively correlated with the plasma glucose response to carbohydrate pre-load(Reference Anderson, Catherine and Woodend57), which is in broad agreement with the present study, although the relationship between the plasma glucose response and energy intake at the buffet meal did not quite achieve statistical significance in the present trial (r − 0·36; P = 0·068). It is conceivable that the effects of plasma glucose on appetite regulation and food intake could have been mediated via acylated ghrelin, as glucose has been shown to modulate ghrelin secretion(Reference Shiiya, Nakazato and Mizuta58–Reference Cukier, Pilichiewicz and Chaikomin60); however, the evidence for this playing a major role in the present study is not particularly strong. While the acylated ghrelin response was lower in the high energy turnover trial, and correlated significantly with carbohydrate balance and the glucose response, it was not related to energy intake at the buffet meal. Although it is possible that this lack of association was a consequence of low statistical power, it is also becoming evident that ghrelin itself exerts glucoregulatory effects(Reference Pusztai, Sarman and Ruzicska61), and thus the direction of causality in the acylated ghrelin–glucose relationship in the present study cannot be definitely stated. In addition, evidence from a number of recent studies has suggested that ghrelin appears to act as a marker of energy status, rather than as a direct stimulator of energy intake(Reference Scrimgeour, Gresham and Giles62, Reference Borer, Wuorinen and Ku63) and our data are consistent with this observation. Thus, further study is required to elucidate how carbohydrate balance and acylated ghrelin and glucose concentrations interact to regulate feeding behaviour under low and high energy turnover conditions.
In conclusion, our findings demonstrate that energy balance under high energy turnover conditions is metabolically different from energy balance under low energy turnover conditions, resulting in a more positive carbohydrate balance. This, in turn, was associated with reduced subsequent ad libitum energy intake. Thus, the present findings provide insight into a potential mechanism by which exercise influences appetite and feeding behaviour, although further study is needed to determine whether these observations extend over the longer term.
Acknowledgements
We thank Dr Nicholas Barwell and Dr Lesley Hall for their clinical assistance with subject screening and on experimental study days and Mrs Heather Collin for technical support.
This project received no external funding.
F. L. B. and J. M. R. G. designed the study, F. L. B. was responsible for data collection under the supervision of J. M. R. G., and all authors contributed to data analysis and interpretation, and writing the manuscript.
The authors have no conflicts of interest relevant to this paper.










