Obesity results in numerous detrimental hormonal and glucose metabolism changes that increase the risk of type 2 diabetes and CVD( Reference Gayet, Bailhache and Dumon 1 – Reference Gastaldelli and Basta 4 ). Postprandial hyperglycaemia, which is associated with obesity, is an independent risk factor for CVD( Reference Node and Inoue 5 – Reference Wascher, Schmoelzer and Wiegratz 7 ). Hyperglycaemia contributes to oxidative stress through the overproduction of reactive oxygen species, which play a critical role in vascular endothelial dysfunction( Reference Node and Inoue 5 , Reference Cosentino, Hishikawa and Katusic 8 ). In addition, obesity directly affects the cardiovascular system by increasing heart rate and cardiac output, promoting cardiac hypertrophy, impairing systolic and diastolic functions, and elevating blood pressure( Reference Van Vliet, Hall and Mizelle 9 , Reference Kotsis, Stabouli and Papakatsika 10 ).
The adipose tissue is an active endocrine organ that secretes adipokines, including leptin and adiponectin, which regulate energy homeostasis, insulin sensitivity, and lipid and carbohydrate metabolism( Reference Clarke, Judd and Donohoue 11 , Reference German, Ryan and German 12 ). Both the amount and distribution of adipose tissue may have a negative impact on health. Body fat stored in the central region of the body (i.e. visceral fat) is more strongly associated with obesity-related disorders than subcutaneous fat, which may be due to differences in the metabolic and endocrine functions of the different adipose depots( Reference Bergman, Kim and Catalano 13 , Reference Leray, Serisier and Khosniat 14 ).
Weight loss is recommended for overweight and obese individuals with the goal of reversing the negative health effects associated with obesity. However, there is some evidence that certain metabolic alterations that increase the possibility of regaining lost weight seem to persist even after weight loss, which may have lasting health consequences( Reference Harrington, Gibson and Cottrell 15 – Reference Makoundou, Pataky and Bobbioni-Harsch 18 ). The time course of changes in metabolic and cardiovascular functions with weight gain and subsequent weight loss is not fully understood and is difficult to determine in humans due to the limitations associated with the manipulation of body weight among the same individuals. Thus, in the present study, an obese dog model was used to determine the effect of weight gain, weight loss and body fat distribution on metabolic and cardiovascular changes. We hypothesised that obesity would result in detrimental changes in metabolic and cardiovascular parameters after short-term obesity (12 weeks), which would worsen with prolonged obesity (32 weeks), but would be improved with weight loss.
Materials and methods
Animals
In the present study, eight beagle dogs, three neutered males and five spayed females, with a mean age of 3·3 (se 0·4) years, were used. The dogs were obtained from Covance Research Products, Inc. or the University of Guelph and were kept in the Western College of Veterinary Medicine at the University of Saskatchewan. The animals were housed in 1·1 × 2·7 m kennels at night, but were housed together during the day with access to outdoor runs, and all the dogs were walked daily. The present study and all procedures used were approved by the Animal Research Ethics Board of the University of Saskatchewan and were carried out in adherence to the Canadian Council on Animal Care guidelines for humane animal use.
The dogs were fed a commercial adult maintenance dry diet (Purina Dog Chow; Nestlé Purina). At baseline, the dogs were fed the diet in amounts based on the National Research Council guidelines( 19 ), which were adjusted to maintain an ideal lean body weight (body condition scores 4–5 on a nine-point scale)( Reference Laflamme 20 ). During the obese phase, the dogs were given unlimited access to food. During the weight-loss phase, access to food was restricted to promote a moderate rate of weight loss of 1–2 % per week, which resulted in an energy intake of 74 (se 2·2) % of the lean body weight maintenance energy requirements. Metabolic and cardiovascular variables were measured at four time points: when the dogs were lean (baseline); after the dogs had been fed ad libitum for 12 weeks (obese 12 weeks); after the dogs had been fed ad libitum for 32 weeks (obese 32 weeks); after weight loss when the body weight of the dogs returned to the baseline body weight levels (17 (se 1)-week weight-loss period). The dogs were considered obese at a body condition score >5 on a nine-point scale( Reference Laflamme 20 ).
Body fat analysis
Total body fat, abdominal body fat and subcutaneous body fat as well as lean body mass were measured by computed tomography (CT) scanning. Scanning was performed at 12 and 32 weeks of obesity as well as after weight loss. A single-slice helical scanner (Tomoscan M; Philips Medical Systems North America) was used for scanning when the dogs were obese for 12 weeks and a 16-slice scanner (Aquilion; Toshiba America Medical Systems, Inc.) was used for scanning during all the subsequent time points. The dogs were fasted overnight and sedated using dexmedetomidine hydrochloride (Dexdomitor® 5 μg/kg; Zoetis). When anaesthesia was required for longer duration during the helical CT, the dogs were sedated using propofol (4 mg/kg, Propofol; Novopharm), intubated and anaesthetised with isoflurane (inhalation maintained at 0·5–3 %, IsoFlo®; Abbott Animal Health). The dogs were placed in dorsal recumbency during scanning at all the time points. After scanning, sedation was reversed with atipamezole hydrochloride (5 μg/kg, Antisedan®; Zoetis).
Transverse digital images were obtained through the thorax and abdomen of each dog for the determination of abdominal body fat area. The abdomen was delineated from the thorax at the level of the diaphragm, and 5 mm slices were analysed using General Electric Advantage Windows Server version 2.0-5.0 with Volume Viewer version 10.3.67 (General Electric Company). Thresholds of − 105/ − 135 Hounsfield units, as determined previously in beagles, were used to identify fat( Reference Ishioka, Okumura and Sagawa 21 ). Abdominal fat was partitioned into visceral fat and subcutaneous fat by drawing a region of interest surrounding the visceral cavity using the clearly visible border of the peritoneal wall( Reference Zhao, Colville and Kalaigian 22 ). Visceral fat and subcutaneous fat were measured at the level of each vertebra by quantifying the volume of fat in two slices in the middle of the vertebra. Total fat was calculated as the sum of visceral fat and subcutaneous fat. Total fat, visceral fat and subcutaneous fat for the entire abdomen were calculated as the sum of the fat values from the sum of the two vertebral slices from cranial thoracic vertebra 13 to caudal lumbar vertebra 7 (i.e. T13 to L7).
Blood collection and analyses
After an overnight fast, the dogs were fed a 20 % glucose solution providing 10 g glucose. The solution was consumed by each dog in < 5 min. The dogs were aseptically catheterised using a peripheral intravenous catheter inserted into the cephalic or saphenous vein. Blood samples (3 ml) were taken for serum collection before feeding (time 0) and 15, 30, 45, 60, 90, 120, 150 and 180 min after feeding. Serum glucose analysis was carried out using a glucose oxidase assay method( 23 ) using reagents obtained from Sigma-Aldrich. Serum insulin concentrations were measured by RIA using a commercially available assay kit (Coat-A-Count Insulin; Siemens) previously validated for dogs( Reference Wolfsheimer, Flory and Williams 24 ). Serum glucagon concentrations were measured by ELISA using a commercially available canine-specific assay kit (Biotang, Inc.). Serum glucose and insulin concentrations were determined at all time points. The trapezoidal method was used to determine the incremental AUC for glucose and insulin( Reference Wolever, Jenkins and Jenkins 25 ). Peak concentration and time to peak concentration for serum glucose and insulin were calculated. Serum glucagon concentrations were measured at time 0 and 60 min after glucose ingestion when the dogs were lean, after 32 weeks of obesity and after weight loss. Change in glucagon concentrations was calculated as follows:
Additional fasting blood samples (10 ml) were taken using collection tubes with EDTA. Plasma leptin, adiponectin and C-reactive protein concentrations were measured using canine-specific commercial ELISA kits according to the manufacturer's instructions (Millipore).
Evaluation of heart function and blood pressure by ultrasonography and oscillometry
All ultrasound measurements were performed and analysed by one individual. As a measure of endothelial function, flow-mediated dilation (FMD) was measured in conscious, unsedated dogs using a SonoSite M-Turbo ultrasound unit with a vascular transducer L38x/10-5 MHz (SonoSite Canada, Inc.) before feeding the glucose solution as well as 60 min after feeding based on previously published methods used by our laboratory and other laboratories( Reference Al-Dissi and Weber 26 , Reference Puglia, Freeman and Rush 27 ). Images were captured at end diastole and analysed using Adobe Premiere Elements 2.0 (Adobe, Inc.) and Image-Pro Express 6.0 (Media Cybernetics, Inc.). FMD was calculated using the following formula:
Change in FMD was calculated as follows:
Echocardiographic techniques were used to assess cardiac structure and function using a SonoSite cardiac transducer P21x/5-1 MHz( Reference Thomas, Gaber and Jacobs 28 ). Measurements were taken after an overnight fast. Two-dimensional ultrasonography was used to measure left ventricular volume using the left parasternal apical two- and four-chamber views in diastole and systole( Reference Lang, Bierig and Devereux 29 ). The inner wall of the left ventricle was traced, and Simpson's rule( 30 ) was used to calculate left ventricular volume in diastole and systole, from which stroke volume, ejection fraction and cardiac output were calculated( Reference Hanton, Geffray and Lodola 31 ).
Two-dimensional guided M-mode echocardiography was used to obtain a right parasternal short-axis view of the heart at the level of the papillary muscles( Reference Lang, Bierig and Devereux 29 ). Image analysis software (Image-Pro Express 6·0. Media Cybernetics, Inc.) was used to measure left ventricular internal dimension and left ventricular free wall (LVFW) thickness in systole and diastole, and fractional shortening was calculated( Reference Hanton, Geffray and Lodola 31 ).
Blood pressure and heart rate were measured in fasted dogs by high-definition oscillometry using the Vet Memo Diagnostic HDO Monitor (S+B medVET) and MDS Win software version 1.4.5.1 (S+B medVET) according to a protocol validated in dogs( Reference Brown, Atkins and Bagley 32 ). The cuff (4·5 × 15 cm) was placed at the tail base. An average of three readings was used to determine diastolic and systolic pressures and heart rate.
Statistical analyses
Data are expressed as means with their standard errors. Before performing all analyses, data were examined for normality and outliers using the Kolmogorov–Smirnov test, Q–Q plots and box plots. Repeated-measures generalised linear model with least significant difference posteriori comparisons were used to compare variables between multiple time points (lean, obese for 12 weeks, obese for 32 weeks and after weight loss). Pearson's correlation coefficients between body fat measurements and metabolic and cardiovascular variables that changed with body weight were determined using combined data obtained at the obese and weight-loss time points. Differences were considered statistically significant at P< 0·05. Analyses were carried out using IBM SPSS Statistics version 20 (International Business Machines Corporation).
Results
Body weight was 9·8 (se 0·6) kg at baseline (i.e. at ideal weight level), 12·1 (se 0·7) kg after 12 weeks of ad libitum feeding, 12·8 (se 0·8) kg after 32 weeks of ad libitum feeding and 10·2 (se 0·7) kg after weight loss. Thus, the body weight of the dogs was 23 (se 3) and 30 (se 5) % above the baseline body weight at 12 and 32 weeks of obesity, respectively. Weight was significantly different between all the time points except between baseline and post-weight loss period. Weight loss was achieved over a period of 17 (se 1) weeks with an average loss of 0·9 (se 0·1) % per week.
Abdominal total fat, visceral fat and subcutaneous fat were measured using CT. Fig. 1 shows representative CT scans at the level of lumbar vertebra 5 in the same dog when it was obese for 12 weeks (Fig. 1(A), left) and after it had undergone weight loss (Fig. 1(A), right). In the raw CT image (Fig. 1(A)), fat appears black. However, the same CT scans are shown in Fig. 1(B) with thresholds of − 105/ − 135 Hounsfield units applied to identify fat, which appears as white, with the dashed lines illustrating how the region of interest surrounding the visceral cavity was selected to permit partitioning of visceral fat and subcutaneous fat. After 12 weeks of obesity, total fat, visceral fat and subcutaneous fat volumes, respectively, were 736 (se 106), 186 (se 30) and 550 (se 94) cm3, which increased to 1016 (se 167), 303 (se 52) and 713 (se 135) cm3 after 32 weeks of obesity (Fig. 1(C)). After weight loss, body fat values were 248 (se 89) cm3 for total fat, 74 (se 27) cm3 for visceral fat and 174 (se 64) cm3 for subcutaneous fat (Fig. 1(C)). Values obtained for all the body fat types were significantly different between all the three time points. No significant differences were observed in the proportion of fat, with visceral fat accounting for 26·6 (se 3·7), 31·1 (se 4·2) and 30·5 (se 3·7) % of the total fat at 12 weeks of obesity, at 32 weeks of obesity and after weight loss, respectively.

Fig. 1 Assessment of abdominal total, visceral and subcutaneous body fat. Representative computed tomography scans at lumbar vertebra 5 of the same dog after 12 weeks of obesity and after weight loss (A) without thresholds applied and (B) with thresholds of − 105/ − 135 Hounsfield units applied. Dashed line shows the partitioning of subcutaneous and visceral body fat regions. (C) Abdominal total, visceral and subcutaneous body fat at 12 (□) and 32 (![]() ) weeks of obesity and after weight loss (
) weeks of obesity and after weight loss (![]() ). Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different within the same group (P< 0·05; repeated-measures generalised linear model with least significant difference posteriori test).
). Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different within the same group (P< 0·05; repeated-measures generalised linear model with least significant difference posteriori test).
The serum glucose, insulin and glucagon responses to the oral glucose tolerance test are summarised in Table 1. Fasting glucose concentrations were significantly higher when the dogs were obese for 12 and 32 weeks than when they were lean, and values returned to the normal levels after weight loss. The AUC for glucose also increased after weight gain. However, after weight loss, the mean glucose AUC was intermediate between the values at baseline and those at the obese phase. Fasting insulin concentrations were significantly higher after 32 weeks of obesity than at baseline. After weight loss, mean fasting insulin concentrations were intermediate between the values at baseline and those at the obese phase. The AUC for insulin was significantly lower after weight loss than during the lean phase. The postprandial change in glucagon (Δglucagon) concentrations was negative when the dogs were lean, but positive when the dogs were obese for 32 weeks (Table 1). Despite this trend, no significant differences were observed in glucagon concentrations among the time points during which measurements were recorded in the present study.
Table 1 Serum glucose, insulin and glucagon responses to the oral glucose tolerance test when the dogs were lean, when they were obese for 12 or 32 weeks, and after they had undergone weight loss (Mean values with their standard errors)
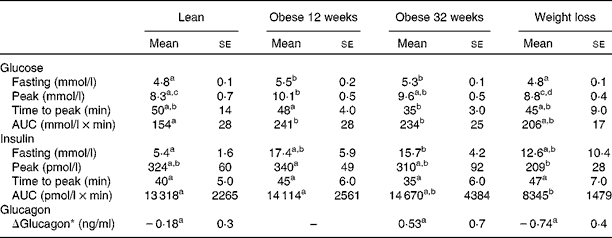
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P< 0·05; repeated-measures generalised linear model with least significant difference posteriori test).
* ΔGlucagon = glucagon 60 min after the glucose challenge − fasting glucagon.
Changes in cardiovascular function were observed with changes in body weight (Fig. 2). FMD was measured before and 60 min after an oral glucose challenge. As postprandial hyperglycaemia is associated with endothelial dysfunction that may worsen with impaired glucose tolerance, ΔFMD was calculated when the dogs were lean, when they were obese for 12 and 32 weeks, and after they had undergone weight loss. ΔFMD was positive when the dogs were lean, but negative when the dogs were obese for 12 and 32 weeks as well as after they had undergone weight loss (Fig. 2(A)). In addition, ΔFMD was significantly lower when the dogs were obese than when they were lean (Fig. 2(A)). Heart rate and cardiac output were 71 (se 3) bpm and 806 (se 69) ml/min, respectively, when the dogs were lean. Heart rate increased to 93 (se 5) bpm (P= 0·003; Fig. 2(B)) and cardiac output also increased to 1256 (se 93) ml/min (P< 0·005; data not shown) after the dogs had been obese for 12 weeks. However, heart rate and cardiac output were not different at baseline, after 32 weeks of obesity or after weight loss.

Fig. 2 Cardiovascular changes in dogs with different body conditions. Measurements were taken when the dogs were lean, when they were obese for 12 or 32 weeks, and after they had undergone weight loss. (A) Flow-mediated dilation (FMD) was measured in dogs before and 60 min after feeding glucose (ΔFMD = FMD at time 60 − FMD at time 0). (B) Heart rate was measured in dogs using high-definition oscillometry. (C) Left ventricular free wall (LVFW) thickness was measured in dogs using two-dimensional guided M-mode ultrasonography. □, Lean; ![]() , obese 12 weeks;
, obese 12 weeks; ![]() , obese 32 weeks;
, obese 32 weeks; ![]() , weight loss. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; repeated-measures generalised linear model with least significant difference posteriori test). bpm, Beats per min.
, weight loss. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; repeated-measures generalised linear model with least significant difference posteriori test). bpm, Beats per min.
Systolic LVFW thickness increased after the dogs had been obese for 12 weeks (P= 0·002; Fig. 2(C)) and decreased significantly after weight loss compared with that observed during the lean phase (P= 0·03). No significant changes were observed in diastolic LVFW thickness, fractional shortening, ejection fraction and stroke volume. Thus, the overall mean values were calculated as 0·75 (se 0·02) cm, 33 (se 1) %, 50 (se 1) % and 12 (se 0·8) ml, respectively. No changes in diastolic blood pressure were observed in response to weight change. However, systolic blood pressure increased from 126 (se 2) mmHg when the dogs were lean to 129 (se 3) mmHg after they had undergone weight loss (P= 0·02), with no changes being observed between the lean and obese states (data not shown).
Plasma leptin concentrations were 1·1 (se 0·7) ng/ml when the dogs were lean, 4·2 (se 1·2) ng/ml after they had been obese for 12 weeks, 4·0 (se 1·4) ng/ml after they had been obese 32 weeks and 2·3 (se 1·1) ng/ml after they had undergone weight loss (Fig. 3(A)). Leptin concentrations were significantly lower when the dogs were lean than when they were obese for 12 weeks (P= 0·01) and 32 weeks (P= 0·03). Plasma adiponectin concentrations when the dogs were lean, were obese for 12 weeks, were obese for 32 weeks and had undergone weight loss were 21·3 (se 4·5), 13·9 (se 2·6), 12·8 (se 2·9) and 10·1 (se 2·2) μg/ml, respectively. Plasma adiponectin concentrations decreased significantly after weight gain and decreased further after weight loss (Fig. 3(B)). Plasma C-reactive protein concentrations did not differ between any of the time points (Fig. 3(C)), and an overall mean concentration value of 413 (se 49) μg/ml was calculated.

Fig. 3 Plasma adipokine and C-reactive protein concentrations in dogs with different body conditions. The plasma concentrations of (A) leptin, (B) adiponectin and (C) C-reactive protein (CRP) were measured when the dogs were lean, when they were obese for 12 or 32 weeks, or after they had undergone weight loss. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; repeated-measures generalised linear model with least significant difference posteriori test). No significant differences were observed in C-reactive protein concentrations.
Correlations between total, subcutaneous and visceral body fat and metabolic and cardiovascular variables were determined using data obtained when the dogs were obese for 12 and 32 weeks and after they had undergone weight loss (Table 2). Fasting glucose concentrations were positively correlated with total fat (r 0·5, P= 0·02) and subcutaneous fat (r 0·5; P= 0·02). Peak glucose concentrations were associated with visceral fat (r 0·4; P= 0·03), but no associations were found between glucose AUC and total, visceral or subcutaneous fat. Insulin AUC (r 0·7, P< 0·001) and peak insulin concentrations (r 0·6, P= 0·001) were positively correlated with visceral fat, but not with total or subcutaneous fat. Significant correlations were found between heart rate and total fat (r 0·5, P= 0·01), visceral fat (r 0·5, P= 0·01) and subcutaneous fat (r 0·5, P= 0·02). Systolic LVFW thickness was more strongly correlated with visceral fat (r 0·6, P= 0·001) than with total fat (r 0·4, P= 0·03) and was not correlated with subcutaneous fat (r 0·3, P= 0·1). No significant correlations were found between total, visceral or subcutaneous fat and plasma leptin or adiponectin concentrations.
Table 2 Correlations between total, subcutaneous and visceral body fat and metabolic and cardiovascular variables using combined data obtained when the dogs were obese for 12 and 32 weeks and after they had undergone weight loss

bpm, Beats per min; FMD, flow-mediated dilation; LVFWs, left ventricular free wall systole.
* ΔFMD = FMD 60 min after the glucose challenge − fasting FMD.
Discussion
The major finding of the present study was the occurrence of significant alterations in glucose, cardiovascular and adipokine parameters after only 12 weeks of obesity in a dog model of obesity. These metabolic and cardiovascular alterations persisted after 32 weeks of obesity, and weight loss reversed some, but not all, of these alterations. These results suggest that detrimental metabolic and cardiovascular changes occur very quickly with weight gain, and because weight loss may not completely reverse these changes, obesity prevention may be vital for promoting long-term health.
In addition, we have demonstrated for the first time in an obese dog model that metabolic and cardiovascular changes are most closely correlated with visceral fat, not with subcutaneous fat, as has been demonstrated in human subjects( Reference Gastaldelli and Basta 4 , Reference Bergman, Kim and Catalano 13 ). The present study supports the negative association between visceral fat and cardiovascular and metabolic markers in dogs, which provides evidence of the suitability of the dog as an obesity model for humans. One of the strengths of the present study is the ability to use the same dogs as their own control, which would be extremely challenging to do using human participants. Thus, the obese dog model is uniquely powerful because it is possible to track the same dogs under all the body states while having strict control over their diet and activity levels. A limitation of the dog as a cardiovascular model for humans is that dogs have HDL as their predominant lipoprotein rather than LDL as humans do. However, obese dogs have been shown to develop lipid abnormalities similar to those observed in insulin-resistant humans( Reference Bailhache, Nguyen and Krempf 33 ). Dogs are also advantageous as a model because unlike rodents, they are large enough to permit the use of the same ultrasound and glycaemic testing techniques as those routinely used in humans.
Weight gain increased fasting glucose concentrations as well as the AUC for glucose, while weight loss resulted in the return of fasting glucose concentrations to the baseline levels, but glucose AUC was intermediate between the lean and obese levels. In other words, fasting glucose concentrations normalised with weight loss after the obese phase, while glucose AUC failed to completely normalise. Both obesity and hyperglycaemia are risk factors for the development of endothelial dysfunction( Reference Kawano, Motoyama and Hirashima 6 , Reference Williams, Chowienczyk and Wheatcroft 34 ), and weight loss may improve postprandial endothelial dysfunction( Reference Haspicova, Milek and Siklova-Vitkova 35 , Reference Kerr, Livingstone and McCrorie 36 ). Hyperglycaemia may induce endothelial dysfunction by initiating pro-inflammatory events such as increases in NF-κB and TNF-α concentrations followed by the overproduction of reactive oxygen species and inhibition of endothelium-dependent NO synthase( Reference Node and Inoue 5 , Reference Cosentino, Hishikawa and Katusic 8 , Reference Mohanty, Hamouda and Garg 37 ). Another mechanism by which hyperglycaemia may contribute to endothelial dysfunction is through the formation of advanced glycation end products( Reference Brouwers, Niessen and Haenen 38 , Reference Dhar, Dhar and Desai 39 ). Methylglyoxal is a reactive glucose metabolite and is a precursor of advanced glycation end products( Reference Desai, Chang and Wang 40 ). We had previously demonstrated that postprandial hyperglycaemia is associated with increased methylglyoxal concentrations and reduced FMD in normal-weight dogs( Reference Adolphe, Drew and Huang 41 ). In the present study, impaired glucose tolerance occurred with obesity and concomitantly postprandial FMD exhibited greater impairment compared with that observed in the lean state, suggesting that elevated blood glucose levels may have a greater impact on endothelial function in obese v. lean individuals. This supports and extends observations in numerous rodent and human studies demonstrating endothelial dysfunction in diabetic individuals( Reference Kawano, Motoyama and Hirashima 6 , Reference Dhar, Dhar and Desai 39 , Reference Bakker, Eringa and Sipkema 42 , Reference Kim, Park and Kim 43 ).
Even though only moderate improvements were observed in glucose AUC after weight loss, AUC for insulin was significantly reduced after weight loss compared with that observed in the baseline lean state. This finding supports the results of other studies that have shown that energy restriction improves pancreatic β-cell function and insulin sensitivity( Reference Leray, Serisier and Khosniat 14 , Reference Larson-Meyer, Heilbronn and Redman 44 , Reference Huffman, Redman and Landerman 45 ). In the present study, dogs were provided about 25 % lesser energy than lean maintenance energy requirements when the oral glucose tolerance challenge was administered after weight loss. Thus, it is possible that energy restriction in addition to the weight loss resulted in the improved insulin response to the glucose challenge.
Glucagon possesses opposing hormonal actions to insulin. It has been suggested that an insulinocentric view of glucose homeostasis is incomplete and that glucagon plays a vital role in the regulation of glucose metabolism( Reference Unger and Cherrington 46 , Reference Unger and Orci 47 ). In the present study, we determined the effect of obesity on serum glucagon concentrations before and 60 min after an oral glucose challenge. Although glucagon concentrations failed to exhibit a postprandial decrease when the dogs were obese, this difference was not statistically significant and requires further exploration to determine the role of glucagon v. insulin in energy fuel metabolism in an obese dog model.
Dogs are considered to be clinically obese when their body weight exceeds 15 % of their ideal body weight( Reference Gossellin, Wren and Sunderland 48 ), which generally corresponds to a score >5 on a nine-point scale of body condition developed and used clinically for dogs( Reference Laflamme 20 ). In the present study, the dogs gained a substantial amount of weight in a relatively short time frame. After 12 weeks of ad libitum feeding, the body weight of the dogs was an average of 23 % above their ideal body weight, which increased further to 30 % after 32 weeks of ad libitum feeding. The dogs gained weight after only 1 week of ad libitum feeding, and the average body weight was already 15 % above the ideal body weight (i.e. already obese), with the weight of five of the eight dogs being at or above this threshold. During the weight-loss period, it took 9 weeks for the average body weight to return to levels below the 15 % obesity threshold.
Obesity, even after weight loss, appears to have lasting effects on energy homeostasis. For example, a study carried out in dogs has found that re-induction of obesity after weight loss occurs more quickly and with a lower energy intake( Reference Nagaoka, Mitsuhashi and Angell 17 ). Another study has shown that post-weight loss maintenance energy requirements are lower than those before weight gain( Reference German, Holden and Mather 49 ). The reason for this increased efficiency in energy metabolism after weight loss is unknown, but the results of the present study indicate that metabolic alterations occur after a 4-month period of weight loss. Notably, adiponectin concentrations were significantly lower after weight loss than during the obese phase and leptin concentrations remained higher than the pre-obesity levels, both of which could have a prolonged effect on energy balance. Studies carried out by German et al. ( Reference German, Hervera and Hunter 50 ) and Wakshlag et al. ( Reference Wakshlag, Struble and Levine 51 ) also found that plasma adiponectin concentrations did not change in obese dogs undergoing weight loss, though Tvarijonaviciute et al. ( Reference Tvarijonaviciute, Tecles and Martinez-Subiela 52 ) found that adiponectin concentrations did increase in dogs following a weight-loss period. Studies carried out in human weight-loss trials have also found conflicting findings( Reference Klempel and Varady 53 ), with some studies demonstrating increases in plasma adiponectin concentrations after weight loss( Reference Heinonen, Laaksonen and Karhu 54 , Reference Christiansen, Paulsen and Bruun 55 ) and some studies demonstrating no effects( Reference Esposito, Pontillo and Di Palo 56 – Reference Claessens, van Baak and Monsheimer 60 ). The time frame over which weight loss is achieved, the degree of weight loss and how long after weight loss adiponectin concentrations are measured are possible explanations for the different observed effects of weight loss on adiponectin concentrations.
Obesity has been reported to be associated with enhanced sympathetic nervous system activity, which has been implicated in the negative effects of obesity on cardiovascular health( Reference Govindarajan, Alpert and Tejwani 61 ). Elevated heart rate is an independent risk factor for cardiovascular morbidity and mortality in healthy humans as well as among those with established cardiac disease( Reference Palatini and Julius 62 ). Increased sympathetic activation and/or reduced parasympathetic control may be responsible for elevated heart rate in obesity( Reference Palatini 63 ). The sympathetic nervous system is strongly influenced by dietary intake. Fasting or energy deprivation reduces, whereas overfeeding stimulates, sympathetic activity. Thus, increased sympathetic or decreased parasympathetic stimulation may explain the increased systolic LVFW thickness and heart rate observed in the present study during the obese phase. In addition, in the present study, it was found that systolic LVFW thickness was reduced after weight loss compared with that observed in the lean state. This change may be due to the energy restriction during the weight-loss period( Reference Gruber, Nink and Nikam 64 ). Although excess loss of cardiac muscle mass could be a concern, this does not appear to be the case in the present study. First, cardiac atrophy is generally associated with undesirable conditions such as cachexia and malnutrition( Reference Gruber, Nink and Nikam 64 ). Second, compared with other studies examining weight loss in dogs( Reference Blanchard, Nguyen and Gayet 65 – Reference Butterwick and Markwell 68 ), the energy restriction followed in the present study (74 % of the maintenance energy requirements) was moderate. Also, the vertebral muscle mass measured in CT scans was not different in dogs in obese v. post-weight loss states (data not shown), arguing against a loss of lean mass. Most importantly, cardiac output and heart rate after weight loss had returned to the baseline (pre-weight gain) levels. Thus, the smaller LVFW thickness after weight loss was sufficient to maintain normal cardiac function in this dog model and appears to be within the normal range( Reference Crippa, Ferro and Melloni 69 ).
In conclusion, the present study provides evidence that detrimental metabolic and cardiovascular consequences occur within 12 weeks of the onset of obesity in an obese dog model and that these changes most strongly correlate with visceral fat, not with subcutaneous fat. In addition, after an average weight-loss period of 17 weeks, most of these changes exhibited improvement, but some had not quite returned to the baseline lean levels. Thus, the results of the present study emphasise the importance of obesity prevention, as after weight loss not all the metabolic indicators had returned to the normal levels and systolic cardiac muscle thickness was reduced compared with the pre-obesity levels, suggesting possible adverse cardiovascular effects of short-term weight loss. These data, along with evidence from other studies showing lasting changes in energy homeostasis after repeated weight-gain/weight-loss cycles, further emphasise the importance of public health initiatives for obesity prevention.
Acknowledgements
The authors thank Dr Suraj Unniappan for his inputs and suggestions on this manuscript. Horizon Pet Nutrition provided non-financial support in the form of feed ingredients and technical help with feed production.
The present study was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada, Saskatchewan Pulse Growers, the University of Saskatchewan Western College of Veterinary Medicine Vitamin Settlement Grant, and the Canadian Foundation for Innovation. The funding agencies had no role in the design and analysis of the study or in the writing of this article. J. L. A. was the recipient of an NSERC Canada Graduate Scholarship (Doctoral).
The authors' contributions are as follows: J. L. A. performed data collection and data analyses and wrote the manuscript; T. I. S. was involved in all radiology aspects of the research; H. C. performed analysis of the CT data; M. D. D. assisted in the experimental design and statistical analyses and provided conceptual advice; L. P. W. was the principal investigator of this project, supervised the project and designed the experiments. All authors discussed the results and implications and edited the manuscript.
The authors received in-kind materials from Horizon Pet Nutrition towards the work reported in the manuscript and projects related to the present study. The authors have no other conflicts of interest to declare.







