Non-alcoholic fatty liver disease (NAFLD) is a common metabolic liver disease which influences over ¼ of the world’s adults(Reference Zheng, Eslam and George1,Reference Younossi2) . NAFLD includes a range of liver conditions spanning from simple steatosis to non-alcoholic steatohepatitis (NASH), advanced fibrosis or cirrhosis. Higher energy food intake and physical inactivity may contribute to the development and progression of NAFLD in genetically predisposed individuals. Patients with NAFLD often have more than one of the individual features of the metabolic syndrome, and insulin resistance provides a common pathogenetic link between both conditions(Reference Abenavoli, Milic and Di Renzo3,Reference Powell, Wong and Rinella4) . It is known that male sex is a risk factor for NAFLD, but the explanation for this association is not entirely understood(Reference Ballestri, Nascimbeni and Baldelli5,Reference Pemmasani, Yandrapalli and Aronow6,Reference Lonardo, Nascimbeni and Ballestri7) .
Abnormal body composition, typically characterised by reduced appendicular skeletal muscle mass as well as increased visceral fat mass, may also adversely affect the risk of NAFLD development and progression(Reference Shida, Akiyama and Oh8,Reference Shida, Oshida and Oh9) . As one of the principal sites of insulin-mediated glucose uptake, a low skeletal muscle mass results in increased whole-body insulin resistance, thereby promoting the possibility of the metabolic syndrome as well as NAFLD(Reference Klip and Pâquet10,Reference Hong, Hwang and Choi11) . Visceral adipose tissue accumulation also leads to increased whole-body insulin resistance and low-grade inflammation, making it an additional risk factor not only for NAFLD and the metabolic syndrome but also for CVD(Reference Després and Lemieux12,Reference Jakobsen, Berentzen and Sørensen13) . The phenomenon of excess adiposity combined with reduced skeletal muscle mass is called sarcopenic obesity(Reference Chen, Liu and Woo14,Reference Cruz-Jentoft, Bahat and Bauer15) . Using bioelectrical impedance, a measurement of the appendicular skeletal muscle mass and the intra-abdominal visceral fat area can be estimated and the appendicular skeletal muscle mass to visceral fat area ratio, known as the SVR index, has been used as a marker that is suggestive of sarcopenic obesity(Reference Kim, Park and Lim16,Reference Xu, Pan and Liang17,Reference Su, Xu and Zheng18,Reference Moon, Yoon and Won19) .
Interestingly, body composition and fat distribution are markedly different between the sexes, with women having more fat around the buttocks and thighs, and men having more fat around the abdomen(Reference Palmer and Clegg20). Women also have approximately two-thirds less skeletal muscle mass and twice as much adipose tissue than men(Reference Janssen, Heymsfield and Wang21). It is well known that male sex is a risk factor for NAFLD, but the explanation for this is uncertain(Reference Ballestri, Nascimbeni and Baldelli5,Reference Pemmasani, Yandrapalli and Aronow6,Reference Lonardo, Nascimbeni and Ballestri7) . It is currently not known whether the aforementioned sex-related differences in body fat and muscle mass distribution may also differentially impact on the association between the SVR index(Reference Shida, Akiyama and Oh8,Reference Kim, Park and Lim16,Reference Xu, Pan and Liang17) and the histological severity of NAFLD.
Therefore, our study tends to estimate whether there is sex difference in the effect of exposure (SVR) on an outcome (histological level of NAFLD) in a cohort of Chinese middle-aged individuals with biopsy-proven NAFLD. If differences in this association do exist between the sexes, improvements in abnormal body composition could provide a new focus for ameliorating NAFLD development and progression.
Materials and methods
Study subjects and design
A total of 1067 adults with presumed NAFLD (on the basis of high values of serum liver enzymes and/or proof of liver steatosis upon imaging tests) were prospectively enrolled at our hospital over 3 years consecutively (December 2016–September 2020). As shown in Fig. 1, among them, 454 subjects were excluded (including 114 with excessive alcohol intake (>70 g/week in women and >140 g/week in men), one with viral hepatitis A, 216 with viral hepatitis B, ten with viral hepatitis C, one with viral hepatitis D, twenty with autoimmune hepatitis, three with drug-induced liver injury; fifty-eight with histological evidence of ≤5 % of hepatocyte steatosis and thirty-one with missing bioimpedance data from InBody 720). As a consequence, a total of 613 middle-aged individuals with biopsy-proven NAFLD were involved in this research.
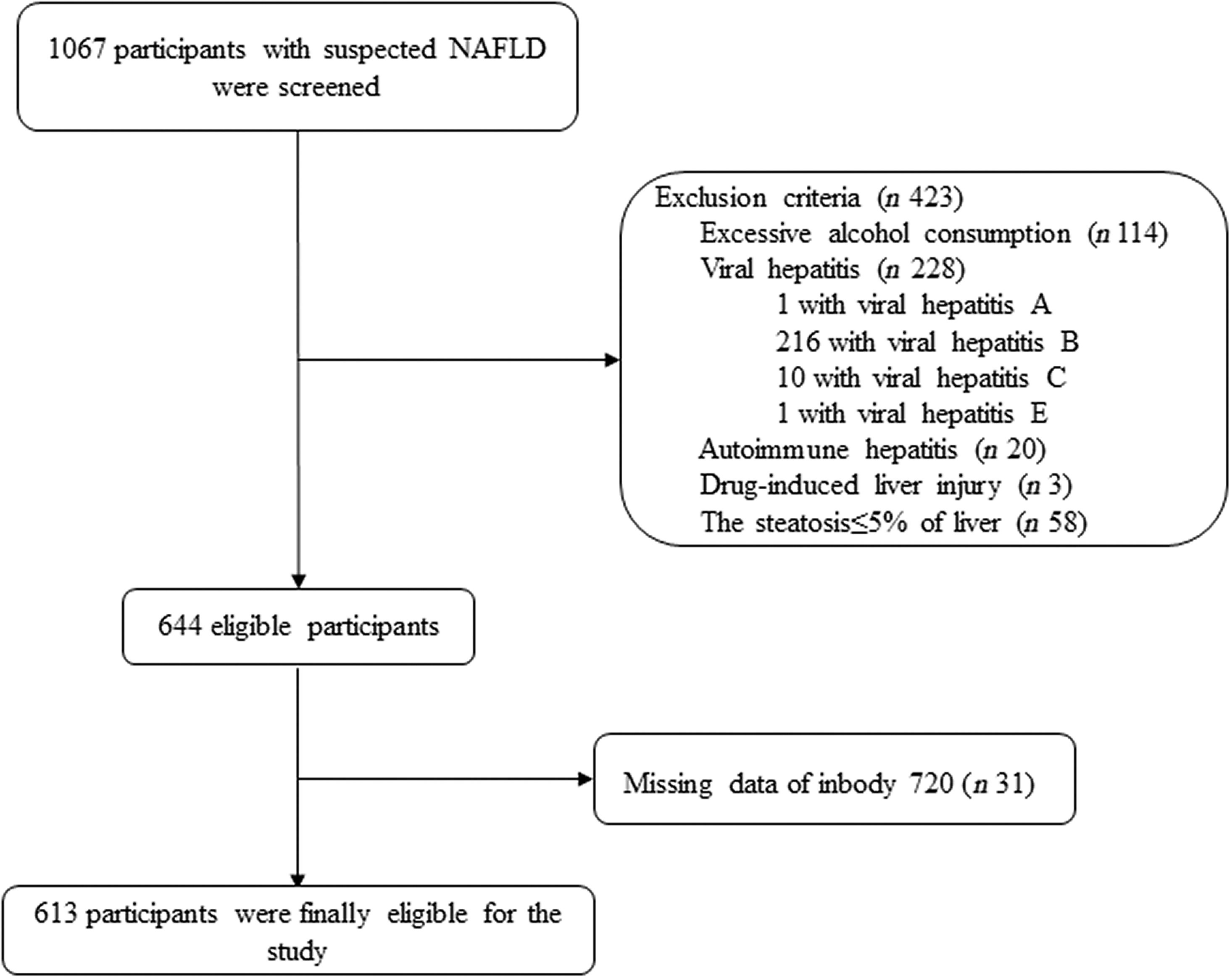
Fig. 1. Flow chart of the study.
The research protocol was approved by the First Affiliated Hospital of Wenzhou Medical University ethical committee (2016-246, 1 December 2016) and recorded in the Chinese Clinical Trial Registry (ChiCTR-EOC-17013562). All procedures conformed with the ethical requirements of the Institutional Research Committee and were in accordance with the 1964 Helsinki declaration. Signed written informed consent was collected from every patient ahead of participation within the research.
Clinical and laboratory data
Anthropometric and laboratory data were collected within 1 d of the liver biopsy procedure, and all of the samples of blood were collected in fasting treating. Standing height as well as body weight was measured, in a condition of the subjects barefoot and wearing light clothing. Samples of venous blood were collected post overnight fasting, with a minimum of 8 h and up to 12 h, followed by analysis at the Clinical Sample Test Room in the hospital. All biochemical parameters were analysed via an automated laboratory analyzer (Abbott AxSYM) with standard methods. BMI was measured as kg over the square of height in metres. BMI ≥ 25 kg/m2 was defined as overweight. Fasting glucose coupled with insulin concentrations was employed for the calculation of homoeostasis model assessment of insulin resistance like following: fasting glucose (mmol/l) × fasting insulin (mU/l)/22·5. Presence of diabetes mellitus was diagnosed by fasting glucose level ≥7·0 mmol/l or HbA1c level ≥6·5 % (≥48 mmol/mol) and/or use of any glucose-lowering drugs. Subjects were considered to have hypertension if their blood pressure was ≥130/85 mmHg or if they were taking any anti-hypertensive drugs. A dual bioelectrical impedance analyzer (InBody 720; Biospace) was employed to measure lean body mass of patient’s limbs and to calculate visceral fat area. According to Heymsfield et al. (Reference Heymsfield, Smith and Aulet22), the addition of the lean soft tissues of arms as well as legs results in appendicular skeletal muscle. We calculated the appendicular skeletal muscle (kg) adjusted by visceral fat area (mm2), or the SVR (an index of sarcopenia obesity expressed as g/mm2).
Liver biopsy
Liver biopsy examination has been described previously(Reference Sun, Zheng and Xu23,Reference Zhou, Ye and Li24) . Briefly, histological evidence of >5 % of steatotic hepatocytes was used as a diagnostic criterion to define NAFLD. Subjects with a NAFLD Activity Score of 5 or greater, and a score of 1 for every one of its three histological components (lobular inflammation, hepatic steatosis and ballooning) were diagnosed as having definite NASH. Stages of hepatic fibrosis were graded from zero to 4, in accordance with the Brunt’s histological criteria(Reference Brunt, Janney and Di Bisceglie25). The presence of liver fibrosis was defined as having a histological stage of 1 or greater.
Statistical analysis
In both men and women, clinical and biochemical data were stratified by tertiles (T) of SVR as follows: T1, <2·18 g/mm2; T2, 2·18–2·63 g/mm2; and T3, >2·63 g/mm2 for men; and T1, <1·50 g/mm2; T2, 1·50–1·88 g/mm2; and T3, >1·88 g/mm2 for women. Categorical variables and continuous strings of data were shown as percentages and means and standard deviation or medians (1st quartile, 3rd quartile), respectively. The one-way ANOVA and the Pearson’s χ 2 test were employed to test significant differences in clinical and biochemical variables among the patient groups. Multivariable logistic regression analyses were used to examine the association between SVR (as the exposure variable) and the histological severity of NAFLD (i.e. presence of NASH or NASH with any stage of liver fibrosis, as the outcome measures) within both women and men. Subgroup and interaction analyses were also employed to test the association between decreasing SVR (as the exposure variable) and the presence of NASH or NASH with any stage of liver fibrosis (as the outcome measures). The likelihood ratio test was used to examine the interactions as well as modifications of different patient subgroups. Statistical significance was examined at two-sided P-value of 0·05. All statistical tests were performed with R software (version 3.5.2, R Foundation for Statistical Computing).
Results
Baseline characteristics of participants
Six hundred and thirteen biopsy-confirmed NAFLD were included, among which 72·3 % (n 443) were men. NASH was confirmed in 257 subjects (men: 176, 68·5 %). The major biochemical as well as clinical features and the liver histology features of participants, stratified by sex as well as SVR tertiles, are illustrated in Table 1. Men in the first tertile of SVR (i.e. reflecting a greater reduction in appendicular skeletal muscle mass:visceral fat area ratio) were more likely to be older and overweight/obese and to have type 2 diabetes than those belonging to the second and third tertiles of SVR. Notably, the former also had a greater proportion of definite NASH, as well as liver fibrosis, lobular inflammation and hepatocyte ballooning on histology. Conversely, women in the first tertile of SVR tend to be older and overweight/obese and to have hypertension than those belonging to the second and third tertiles of SVR. However, no significant differences were found in definite NASH and all its individual histological scores across SVR tertiles in women.
Table 1. Baseline characteristics of patients with biopsy-proven non-alcoholic fatty liver disease (NAFLD), stratified by sex and appendicular skeletal muscle mass:visceral fat area ratio tertiles
(Numbers and percentages; mean values and standard deviations)
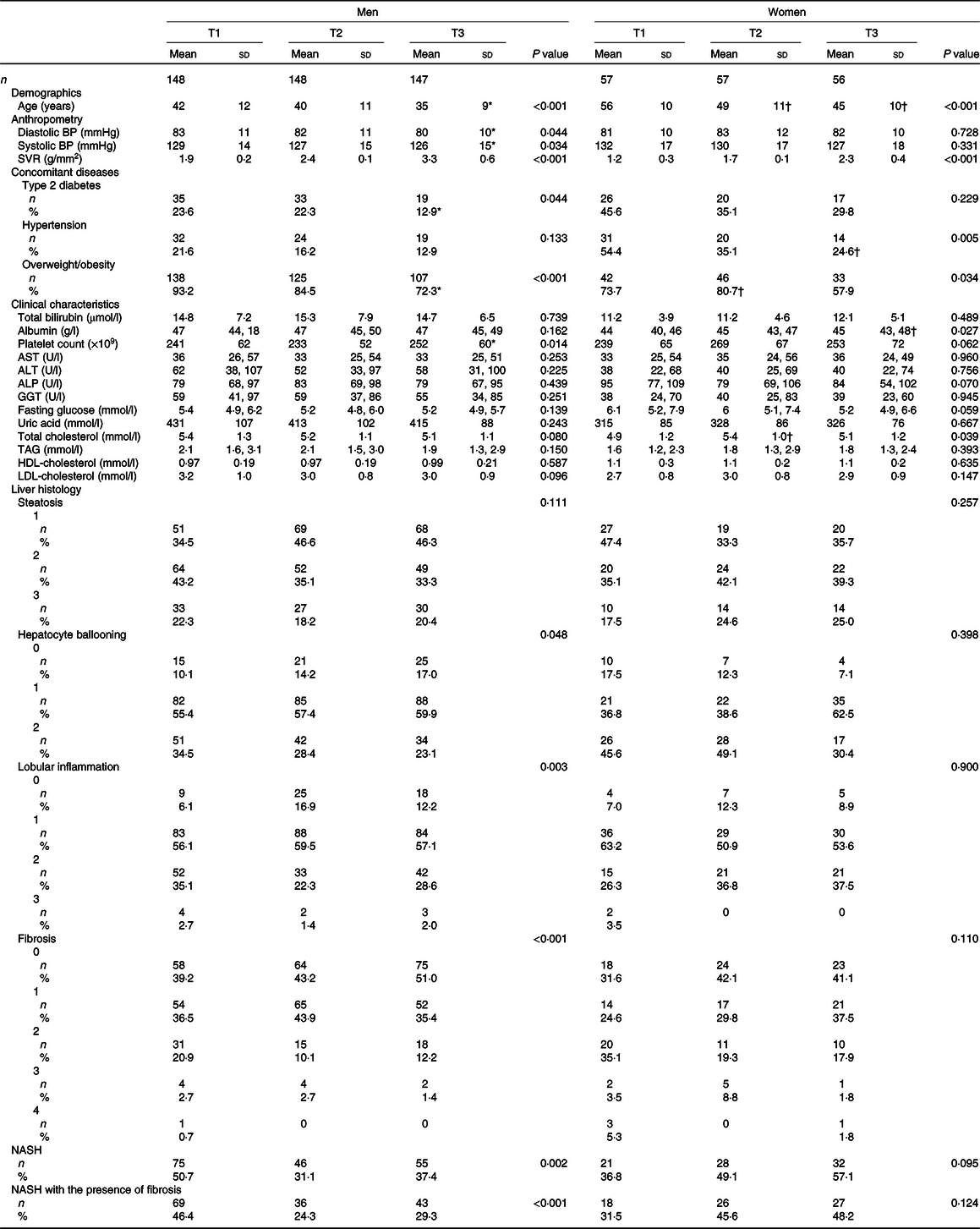
BP, blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyltransferase; NASH, non-alcoholic steatohepatitis.
* P value < 0·05, v. T1 in men.
† P value < 0·05, v. T1 in women.
Association between appendicular skeletal muscle mass to visceral fat area ratio and severity of non-alcoholic fatty liver disease
Multivariable logistic regression analyses were used to estimate the effect of SVR (as the exposure variable) on the severity of NAFLD (as the outcome measures). As shown in Table 2, SVR showed a significant inverse association with either NASH (OR 0·77, 95 % CI 0·61, 0·91; P = 0·022) or NASH with the presence of any stage of liver fibrosis (OR 0·72, 95 % CI 0·56, 0·91; P = 0·006) in unadjusted logistic regression models. However, these associations were no longer significant after adjustment by sex, age, BMI, hypertension, pre-existing type 2 diabetes and other potential confounding factors (adjusted models 2).
Table 2. Associations between appendicular skeletal muscle mass to visceral fat area ratio (SVR) and severity of non-alcoholic fatty liver disease (NAFLD) in the whole cohort of patients*
(Odds ratio and 95 % confidence intervals)
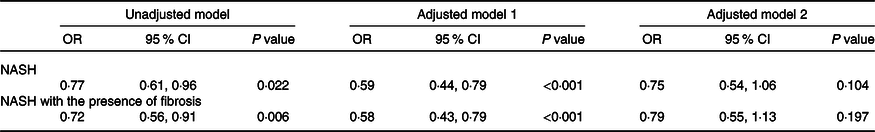
NASH, non-alcoholic steatohepatitis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMO-IR, homoeostasis model assessment of insulin resistance.
* Data are expressed as OR and 95 % CI tested by logistic regression analysis. Model 1 was adjusted for age and sex; Model 2 was adjusted for age, sex, BMI, serum albumin, AST, ALT, HDL-cholesterol, LDL-cholesterol, TAG, total cholesterol, HOMA-IR score, hypertension and type 2 diabetes.
Subgroup analyses for the relevance of appendicular skeletal muscle mass to visceral fat area ratio with severity of non-alcoholic fatty liver disease
As detailed in Table 3, there were significant interactions of SVR with sex (P interaction = 0·017 for NASH, P interaction = 0·033 for NASH with the presence of fibrosis) and age (P interaction = 0·009 for NASH, P interaction = 0·033 for NASH with the presence of fibrosis), but not with overweight/obesity, hypertension or pre-existing diabetes (all P interaction > 0·05). In stratified analyses, after adjustment for potential confounders, SVR remained inversely relevant with the severity of NAFLD only within men (adjusted OR 0·62, 95 % CI 0·42, 0·92, P = 0·017 for NASH; adjusted OR 0·65, 95 % CI 0·43, 0·99, P = 0·043 for NASH with the presence of fibrosis), and in older individuals (adjusted OR 0·40, 95 % CI 0·22, 0·74, P = 0·003 for NASH; adjusted OR 0·46, 95 % CI 0·25, 0·86, P = 0·014 for NASH with the presence of fibrosis).
Table 3. Adjusted associations between appendicular skeletal muscle mass to visceral fat area ratio (SVR) (as the exposure variable) and severity of non-alcoholic fatty liver disease (NAFLD) (as the outcome measure) in different patient subgroups*
(Odds ratio and 95 % confidence intervals)
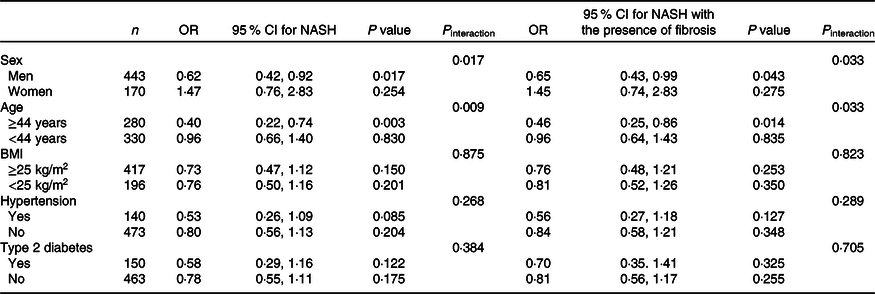
NASH, non-alcoholic steatohepatitis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMO-IR, homoeostasis model assessment of insulin resistance.
* All models and OR (95 % CI) have been estimated using a logistic regression. SVR was included as a continuous measure. All logistic regression models (reported above) were adjusted for age, sex, BMI, serum albumin, AST, ALT, HDL, LDL, TAG, total cholesterol, HOMA-IR score, hypertension and type 2 diabetes (with the exception of the specific variable used for stratifying each patient subgroup).
Discussion
We found that there are clear sex-related and age-related associations between a progressive reduction in SVR (i.e. reflecting a reduction in appendicular skeletal muscle mass to visceral fat area ratio) and the severity of NAFLD histology. In particular, we found that decreasing SVR is closely associated with NASH in both men and older individuals, but not in women or younger subjects. Notably, the interaction test between SVR and sex was significant for either definite NASH or NASH with the presence of any stage of liver fibrosis. According to our knowledge, this is the largest and first research to date that has investigated the impact of sex on the association of SVR as well as disease severity of liver for patients having NAFLD.
Over the past few years, the relationship between decreasing SVR and presence of cardiometabolic diseases (including NAFLD) has attracted increasing scientific interest(Reference Shida, Akiyama and Oh8,Reference Kim, Park and Lim16,Reference Xu, Pan and Liang17,Reference Su, Xu and Zheng18,Reference Shi, Chen and Qiu26) . However, no unified conclusion has been reached mainly due to different diagnostic methods of NAFLD and sarcopenic obesity. According to a cross-sectional research of Japanese individuals, Shida et al. published that SVR was inversely associated with NAFLD for both sexes; however, in this research, the determination of NAFLD was obtained by ultrasonography and not by biopsy(Reference Shida, Akiyama and Oh8). Another cross-sectional study performed in Chinese patients with type 2 diabetes found that SVR was inversely associated with the existence of ultrasonography-defined NAFLD only for women(Reference Su, Xu and Zheng18). An independent association of sarcopenia with both NAFLD and NAFLD-related advanced fibrosis has been recently reported in a meta-analysis of three cross-sectional studies(Reference Tovo, Fernandes and Buss27). And, Seo et al. found that sarcopenia, as estimated from bioimpedance measurements, was related to a greater risk of having ultrasound-defined NAFLD only for men, in a large cross-sectional research from the Seoul Metabolic Syndrome Cohort(Reference Seo, Lee and Park28). Koo et al. claimed that sarcopenia was related to NAFLD, but this association became non-significant after adjustment for potential confounding elements. Among subjects with biopsy-proven NAFLD, sarcopenic patients tend to have NASH compared with their counterparts without sarcopenia(Reference Koo, Kim and Joo29). However, these investigators did not perform separate statistical analyses stratifying by sex.
It is known that when skeletal muscle mass decreases and visceral adipose tissue increases, insulin-mediated skeletal muscle and adipose tissue’s ability to use or store blood glucose decrease. Indeed, low skeletal muscle mass and increased visceral fat accumulation reduce whole-body insulin-mediated glucose uptake and may promote the development of NAFLD(Reference Shida, Akiyama and Oh8,Reference Kuhl, Hilding and Ostenson30) . In our study, we found that the association between decreasing SVR and NASH (with or without accompanying liver fibrosis) was observed only in men and in older individuals (age ≥ 44 years). A possible explanation for this observed sex-related difference in the association between decreasing SVR and the severity of NAFLD histology is that there are sex differences in muscle pathophysiology as well as body fat deposition that might confound the association between SVR and whole-body insulin resistance. Sex differences in muscle capillary density, muscle fibre type composition and oestrogen receptors expressed in skeletal muscle may affect the ability of glycolytic v. oxidative substrate metabolism(Reference Lundsgaard and Kiens31). Additionally, women have more subcutaneous adipose tissue than men, which may affect circulating levels of adipokines. Circulating adipokines adversely affect skeletal muscle metabolism through receptor binding(Reference Lundsgaard and Kiens31,Reference Schautz, Later and Heller32) . There is also evidence showing that appendicular muscle mass is inversely related to HOMA-estimated insulin resistance for regular-weight as well as obese men, however not for women(Reference Kim33), and that sarcopenic obesity occurs more frequently in older individuals, who lose muscle mass and become more centrally obese and directly affected by inflammation and insulin resistance with advancing years of life(Reference Zamboni, Mazzali and Fantin34).
Our research has several significant limitations which should be listed. First of all, sarcopenia not only includes lower muscle mass but also includes lower hand-grip strength and gait speed. A confirmed diagnosis of sarcopenic obesity needs to include measures of both muscle mass and muscle function, but these latter were not measured in our study(Reference Chen, Liu and Woo14,Reference Cruz-Jentoft, Baeyens and Bauer35) . Second, due to the cross-sectional design of the research, a causal and temporal relationship cannot be established. And then, the gold standard method for measuring visceral fat area, i.e. the computed tomography, was not available in our study because of its high monetary cost as well as radiation exposure to each patient. However, we used a dual bioelectrical impedance analyzer, which is a reliable, non-radioactive as well as repeatable methodology. A good correlation has been reported between computed tomography scans and bioelectrical impedance analyzer for assessing abdominal visceral adiposity(Reference Ida, Hirata and Odori36). Finally, we did not include any detailed information about physical activities, menopausal status, sex hormone levels or current use of oestrogen and progestogen drugs.
To conclude, the findings of our research showed that a progressive appendicular skeletal muscle mass:visceral fat area ratio (as reflected by decreasing SVR) is strongly associated with the severity of NAFLD (i.e. NASH with varying levels of fibrosis) only for men, but not for women, after being adjusted for potential confounding factors. These findings provide a new focus for ameliorating NAFLD development and progression. However, future prospective and mechanistic researches are required to identify the linkage of sarcopenia obesity with the risk and progression of NAFLD better.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82070588), High Level Creative Talents from Department of Public Health in Zhejiang Province (S2032102600032), Project of New Century 551 Talent Nurturing in Wenzhou. G. T. is supported in part by grants from the University School of Medicine of Verona, Verona, Italy. C. D. B. is supported in part by the Southampton NIHR Biomedical Research Centre (IS-BRC-20004), UK.
Study concept and design: G. L. and M. -H. Z. Acquisition of data: G. L., Y. Y., X. -X. W., H. -L. M., L. -J. T., O. -Y. H. and X. -Y. P. Pathology analysis: Y. J. Drafting of the manuscript: G. L., R. S. R. and M. -H. Z. Critical revision: K. I. Z., G. T. and C. D. B. Statistical analysis: G. L. Study supervision: M. -H. Z. All authors contributed to the manuscript for important intellectual content and approved the submission.
The authors declare that there are no conflicts of interest associated with this manuscript.






