Vitamin D deficiency is an increasingly recognised global health problem( Reference Adams and Hewison 1 ). Studies have shown that vitamin D plays important roles in regulating Ca and P metabolism and in maintaining normal bone mineral salts in the human body( Reference Holick 2 ).
Vitamin D deficiency increases the risks of osteomalacia, fracture and fall( Reference Bischoff-Ferrari, Willett and Wong 3 , Reference Bischoff-Ferrari, Dawson-Hughes and Staehelin 4 ). Vitamin D and Ca supplementation decrease the incidence of hip fracture and other non-vertebral fractures( Reference Michael, Whitlock and Lin 5 , Reference Del Valle, Yaktine and Taylor 6 ). However, the proper concentrations of serum 25-hydroxyvitamin D (25OHD) that are required for adequate bone health have been debated and remain controversial.
Serum 25OHD concentrations associated with plateauing of serum parathyroid hormone (PTH) vary between studies. A limited number of studies that have investigated vitamin D supplementation indicate that the serum 25OHD concentrations leading to suppression of PTH are about 50 nmol/l( Reference Lips 7 , Reference Frost, Abrahamsen and Nielsen 8 ). However, other studies have shown that PTH reaches a nadir at higher serum 25OHD concentrations, approximately 75–80 nmol/l( Reference Lips, Hosking and Lippuner 9 , Reference Chapuy, Preziosi and Maamer 10 ). The US population-based National Health and Nutrition Examination Survey III study shows that BMD of the hip is increased with a higher serum 25OHD of up to 50( Reference Wicherts, van Schoor and Boeke 11 ) or 80 nmol/l( Reference Lips, Hosking and Lippuner 9 ). The relationships between serum 25OHD, PTH and bone density status are often inconsistent and influenced by predictors such as seasonality, age, ethnicity, BMI, physical activity levels and genetic factors( Reference Zhao, Zhou and Bu 12 – Reference Lucas, Ponsonby and Dear 14 ). Therefore, it is necessary to better understand the contributions of serum 25OHD concentrations in order to determine factors that may influence the status of bone density. The present study aims to estimate whether serum 25OHD concentrations are associated with serum PTH and BMD in urban males living in Guiyang, Southwest China.
Methods
Study sample
We performed a cross-sectional study in a community, in Guiyang, Southwest China, from November 2009 to February 2010. The study population was from the Guiyang Health Measures Survey, which has been set up as a prospective observational study aiming to evaluate chronic diseases in Guiyang population. In brief, a random sample of men aged between 20 and 79 years and who lived in Guiyang City (26.57°N) for more than 10 years were invited to participate by examination notice or a home visit. After a main interview (n 700), participants were invited to the hospital of Zaiji Community, where blood samples were collected from 634 subjects. Serum concentrations of 25OHD, PTH, Ca, P and creatinine (Cr) were measured in 634 subjects. BMD measurements of the lumbar spine, femoral neck and total hip were obtained in a sub-sample including 505 participants. The 634 subjects were stratified into three groups according to their age: 346 subjects in the young age group (20–39 years), 182 subjects in the middle-aged group (40–59 years) and 106 subjects in older age group (60–79 years). All the analyses were conducted by intensively trained and supervised interviewers, and were tape-recorded to monitor the quality of the data. Informed consent was obtained from all the subjects. The study protocol has been approved by the local Medical Ethics Committee of the Guiyang Medical College.
Clinical and biochemical measurements
All the subjects completed a standard questionnaire on socio-economic characteristics (e.g. education, occupation), lifestyle factors (e.g. outdoor activity, smoking status, alcohol consumption) and medical history. Educational status was assessed by asking the subjects for the highest educational level completed, and ranged from primary school to university. Smoking status and alcohol consumption were classified as ‘never’ and ‘current’ (smoking or drinking regularly in the past 6 months). Medical history included chronic obstructive pulmonary disease, chronic renal disease, CVD, stroke, peripheral arterial disease, diabetes, malignant neoplasms and joint disorders including osteoarthritis and rheumatoid arthritis. All the participants completed anthropometrical measurements with the assistance of trained staff using standard protocols. BMI was calculated as weight in kilograms divided by height in metres squared (kg/m2). Exclusion criteria were as follows: presence of known bone disease, liver or renal disease (values ≥2×upper limit of normal for aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, urea, Cr and uric acid resulted in exclusion), serious diseases (e.g. cancer) and use of medication influencing bone metabolism (excluding Ca and vitamin D supplementation). Blood samples were obtained in the morning after fasting for 8–10 h and were immediately centrifuged; the separated serum samples were frozen for 25OHD and PTH measurements. Serum 25OHD concentrations and PTH concentrations were measured by automatic RIA (DiaSorin Inc.). The lower and upper detection limits of serum 25OHD concentrations and PTH concentrations were 4·1–149·1 nmol/l and 1·37–5·68 pmol/l, respectively. The inter-assay CV of serum 25OHD and PTH were 8·8 and 2·4 %, respectively. The intra-assay CV of serum 25OHD and PTH were 11·1 and 4·9 %, respectively. Serum Ca, P and Cr levels were measured using an automated biochemical analyzer (Olympus AU5400; Olympus). The analyses were carried out at the Endocrine Laboratory of the Guiyang Medical College Center.
Bone mineral density measurement and definition of osteoporosis
The dual-energy X-ray absorption scans were obtained using a lunar prodigy scanner (GE Medical System Co. Ltd). The lumbar spine and left hip were scanned. BMD (g/cm2) of the femoral neck, total hip and lumbar spine (L1–L4) were measured. Osteoporosis was defined according to the WHO classification( Reference Kanis, Melton and Christiansen 15 ).
Statistical analyses
Statistical analyses were performed using SPSS 13.0 for Windows (SPSS). Continuous variables were presented as mean values and standard deviations except for skewed variables, which were presented as medians and interquartile ranges. Categorical variables were expressed as numbers (proportions). We used ANCOVA, which was adjusted for potential confounders (such as age, educational status, Cr levels, chronic disease, smoking status). Serum 25OHD (nmol/l) was presented as quartiles: quartile 1, <25 nmol/l; quartile 2, 25–<50 nmol/l; quartile 3, 50–<75 nmol/l; and quartile 4, ≥75 nmol/l. Subsequently, locally weighted regression smoothing (LOESS) plots with 95 % CI were obtained in S-Plus (MathSoft Inc.) to investigate the relationship between serum 25OHD and various outcome measures. Differences among groups were tested by one-way ANOVA and post hoc comparisons were performed using Bonferroni correction. Comparisons between categorical variables were performed using the χ 2 test. The unadjusted and multivariate-adjusted logistic regression analyses were used to assess the risk of potential confounders (as age, educational status, Cr levels, chronic disease, smoking status) in relation to each quartile increase in serum 25OHD concentration. All the statistical tests were two-sided, and a P value<0·05 was considered to be statistically significant.
Results
Baseline characteristics of the population are described in Table 1. Mean values of serum 25OHD, PTH and BMI were significantly higher in the middle-aged and older-aged groups than in the young-aged group. As expected, serum Ca and P concentrations and BMD values of the femoral neck and total hip measurements were significantly higher in the young-aged group than in the middle-aged and older-aged groups (Table 2). A negative correlation between serum 25OHD and PTH concentrations was observed in all subjects (r −0·207, P=0·003). Table 3 shows values of serum Ca, P, PTH, BMD among different 25OHD concentration groups, as well as different age groups in the study. Serum 25OHD was <25 nmol/l in 16·1 % of the subjects, <50 nmol/l in 59·3 % of the subjects, <75 nmol/l in 87·4 % of the subjects and >75 nmol/l in 12·6 % of the study participants. In all age groups, the mean serum concentration of PTH was negatively correlated with 25OHD. In contrast, serum Ca and P concentrations did not differ significantly from each group. All the BMD values were greater in the higher serum 25OHD groups, whereas only total hip in the young age group, total hip and L1–L4 (P=0·041, 0·036) in the middle-aged group and total hip in the older age group (P=0·024) showed statistical significance (P=0·023).
Table 1 Baseline characteristics (Numbers and percentages; mean values and standard deviations)Footnote *
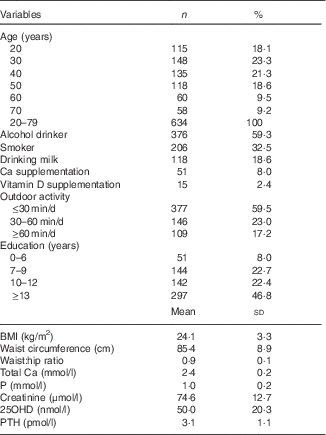
25OHD, 25-hydroxyvitamin D; PTH, parathyroid hormone.
* For conversion of 25OHD from nmol/l to ng/ml, divide by 2·496. For conversion of PTH from pmol/l to pg/ml, multiply by 11·1. Serum 25OHD was assessed in 634 subjects and PTH was assessed in 516 subjects.
Table 2 Mean values of BMI, serum 25-hydroxyvitamin D (25OHD), parathyroid hormone (PTH), calcium, phosphorus and bone mineral density (BMD) in different age groups (Mean values and standard deviations)Footnote *
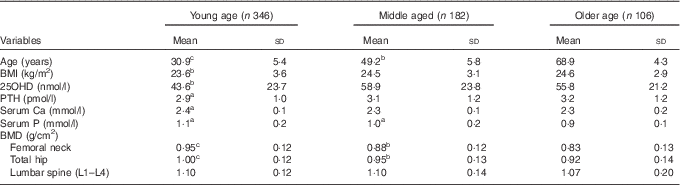
* For conversion of 25OHD from nmol/l to ng/ml, divide by 2·496; for conversion of PTH from pmol/l to pg/ml, multiply by 11·1. a P<0·05, b P<0·01, c P<0·001, as compared with the reference category older age group.
Table 3 Differences in the mean values of bone mineral density (BMD), parathyroid hormone (PTH) and serum calcium and phosphorus in different serum 25-hydroxyvitamin D (25OHD) concentration groups among age groups (Mean values and standard deviations)

* For conversion of 25OHD from nmol/l to ng/ml, divide by 2·496; for conversion of PTH from pmol/l to pg/ml, multiply by 11·1. Adjusted for age, level of education, BMI, creatinine levels and smoking status. BMD measurements of the lumbar spine, femoral neck and total hip were obtained from a sub-sample including 505 participants. a P<0·05, b P<0·01, c P<0·001, as compared with the reference category >75 nmol/l.
Univariate analyses, after adjusting for age, educational status, Cr levels, chronic disease and smoking status, have been described in the Methods section. Our results showed that a significant positive association was found between 25OHD concentrations and BMD of the total hip (r 0·289, P=0·022) and L1–L4 (r 0·179, P=0·031) in the young group, of the total hip (r 0·157, P=0·035) and the femoral neck (r 0·149, P=0·038) in the middle-aged group and of the total hip (r 0·154, P=0·04), femoral neck (r 0·254, P=0·017) and lumbar spine (L1–L4) (r 0·260, P=0·011) in the older age group in our study.
The relationships among 25OHD, BMD and PTH are shown in Fig. 1. The LOESS plots with 95 % CI, which were adjusted for age, BMI, Cr levels and smoking status, are also shown in Fig. 2. We found that the relationship between 25OHD and BMD of the total hip and the L1–L4 vertebrae in all groups reached a plateau in the range of 30–50 nmol/l as shown in Fig. 2(a)–(d).

Fig. 1 Relationship between serum 25-hydroxyvitamin D (25OHD) and parathyroid hormone (PTH) (P=0·003).

Fig. 2 Relationship between serum 25-hydroxyvitamin D (25OHD) concentrations and bone mineral density (BMD) values of total hip in the young group (a, r 0·189, P=0·025), middle-aged group (b, r 0·149, P=0·040) and older age group (c, r 0·022, P=0·019), BMD of the lumbar spine (L1–L4) in the middle-aged group (d, P=0·038). All plots are adjusted for level of education, level of BMI, creatinine levels and smoking status. The ![]() represents the 95 % CI.
represents the 95 % CI.
Discussion
It is well established that vitamin D deficiency causes secondary hyperparathyroidism and bone loss. The results of the Longitudinal Ageing Study in Amsterdam confirmed that low serum 25OHD concentrations are associated with an increase in serum PTH, increased bone turnover and lower BMD( Reference Chapuy, Preziosi and Maamer 10 ). However, the threshold values at which serum PTH and bone resorption started to increase and BMD started to decrease were highly variable in different studies.
In our study, compared with participants with higher serum 25OHD concentrations, those with lower 25OHD concentrations combined with higher serum PTH concentrations were older and were less commonly current alcohol consumers and current smokers. Comparatively, Li et al.( Reference Li, Yin and Yao 16 ) investigated the associations of serum 25OHD concentrations with hypertension among 1420 participants, aged 20–83 years in Dali (25°N). In their study, the mean concentration of 25OHD was 22·0 ng/ml (55 nmol/l). Moreover, another study showed that 939 Chinese old men in Hong Kong (22°N)( Reference Chan, Chan and Woo 17 ) had a mean serum 25OHD concentration of 77·9 nmol/l. The different levels of 25OHD that were observed among the three Chinese studies may have resulted from the different study populations, geography and season. In the present study, serum PTH reached a plateau at lower values of serum 25OHD of 50 nmol/l, which may imply that the optimal serum 25OHD concentration is approximately 50 nmol/l for bone health. Furthermore, a significant positive association between 25OHD concentrations and BMD of different parts was found, and BMD increased to a higher value with higher values of 25OHD concentration about 50 nmol/l. These results indicate that the optimal serum threshold of 25OHD for bone health should be between 30 and 50 nmol/l. This study is consistent with previous studies on the relationship of serum 25OHD with BMD as well as serum 25OHD with serum PTH in older subjects( Reference Wicherts, van Schoor and Boeke 11 , Reference Kuchuk, Pluijm and van Schoor 18 , Reference Bates, Carter and Mishra 19 ) or in the young age and the middle-aged groups( Reference Frost, Abrahamsen and Nielsen 8 ).
Recent discussions have focused on circulating concentrations of 25OHD, which is appropriate. This question is important because it has implications for the prevention of osteoporosis and fractures, and it has relevance for public health strategies, including food fortification with vitamin D and the use of supplements. The required serum 25OHD concentrations have usually been established by assessing the threshold serum concentration of 25OHD, below which serum PTH starts to increase. It is suggested that the simultaneous measurement of serum PTH may aid in interpreting the circulating concentrations of serum 25OHD because of an inverse relationship between them. However, the increase of serum PTH associated with vitamin D deficiency is usually within the normal reference range. In addition, serum PTH has a short half-life and depends on Ca intake, and thus different data sets could lead to different conclusions( Reference Jorde, Sneve and Hutchinson 13 ). This threshold concentration of serum 25OHD may be varied, from 37·5 nmol/l in a study of American hospital inpatients( Reference Thomas, Lloyd-Jones and Thadhani 20 ), 50 nmol/l in the vitamin D supplementation study of the USA( Reference Malabanan, Veronikis and Holick 21 ) and 75 nmol/l in the French SU.VI.MAX (SUpplémentation en VItamines et Minéraux Anti-oXydants) study( Reference Chapuy, Preziosi and Maamer 10 ). The tendency to establish the required serum 25OHD concentrations by assessing the threshold below which serum PTH starts to rise could lead to the use of almost the upper limit of serum 25OHD concentrations in different data sets as a threshold, establishing the required serum 25OHD concentration range from 50 to 100 nmol/l( Reference Chapuy, Preziosi and Maamer 10 , Reference Kuchuk, Pluijm and van Schoor 18 ). In our study, this threshold is in the range of 30 and 50 nmol/l. A significant positive association between 25OHD concentrations and BMD was observed( Reference Bischoff-Ferrari, Dietrich and Orav 22 ), whereas a threshold of about 80 nmol/l is found. This concentration represents serum 25OHD concentrations above which BMD increased slowly( Reference Bischoff-Ferrari, Giovannucci and Willett 23 ). A review suggests that a desirable serum 25OHD concentration for optimal health begins at 75 nmol/l, with the optimal concentration being 90–100 nmol/l( Reference Kuchuk, Pluijm and van Schoor 18 ). Our study yields a lower threshold (50 nmol/l), which may be due to different assays for determining serum 25OHD( Reference Souberbielle, Friedlander and Kahan 24 ), different regions or different race/ethnicity in the context of vitamin D receptor gene polymorphisms. Different concentrations of serum 25OHD for serum PTH and BMD may also be explained by the extra-renal hydroxylation of 25OHD to the active metabolite 1,25-dihydroxyvitamin D3 in different organs, because it is known that 1,25-dihydroxyvitamin D is capable of acting via both autocrine and paracrine mechanisms( Reference Bischoff-Ferrari 25 ).
An important question relates to the optimum oral intake of vitamin D required to maintain bone health. A review on this matter suggests that all adults aged 50–70 and older than 70 years require vitamin D of at least 15 and 20 µg/d (600 and 800 IU/d), respectively, to maximise bone health and muscle function( Reference Holick, Binkley and Bischoff-Ferrari 26 , Reference Elamin, Abu Elnour and Elamin 27 ). As shown in a meta-analysis on vitamin D status and fracture prevention, a dose of 17·5–20 µg/d (700–800 IU/d) appears to reduce the risk of hip and any non-vertebral fractures in older subjects( Reference Lips, Duong and Oleksik 28 ). Therefore, supplementation dose should be 15–20 µg/d (600–800 IU/d) while sunshine exposure is insufficient. A final executive decision on this question should be made on an individual basis with respect to baseline serum 25OHD concentrations, lifestyle and of course diet.
There are several strengths to our study, including the cohort design, which includes different age groups of male subjects, who are also a representative group of the Southwest China urban population, the combination of serum PTH and measurement of different areas of bone density. However, our study also has its limitations. For example, we measured only serum 25OHD and PTH in a single season. It can be questioned whether serum 25OHD and PTH concentrations measured at a single season reflect the long-term status. In addition, bone turnover markers were not measured.
In conclusion, our study demonstrates that low serum 25OHD concentrations are very common in adult males in the Southwest China urban population. Bone health in the participants is likely to improve when serum 25OHD is raised to concentrations of 30–50 nmol/l. The implication for our study population is that at least 59·3 % should receive vitamin D supplements because their serum 25OHD concentrations are <50 nmol/l.
Acknowledgements
The authors thank the strong support given to us by the health bureau of Yunyang district in Guiyang, sub-district office and the hospital of Zhaiji community, the biochemistry department, the central lab and the endocrinology department of the affiliated hospital of Guiyang Medical College.
Financial support was received from the Government Foundation of Guizhou Province of China.
Q. Z. drafted the article, acquired the data and wrote the article. N. P. designed the questionnaire and implemented the study. Q. Z., S. X. and M. Z. analysed the data. S. X., M. Z., S. Z., H. L., H. Z., M. G., D. W. and R. W. implemented the research and collected data. L. S. is the guarantor of this work and, as such, had full access to all the data of the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All the authors approved the final version of the manuscript and performed the literature search.
All the authors declare that they have no conflicts of interest.








