In recent decades, the incidence of gastric cancer (GC) has tended to decrease, especially in developed countries. Nevertheless, it is still an important cause of the global cancer burden. According to GLOBOCAN 2018(Reference Bray, Ferlay and Soerjomataram1), GC ranked as the fifth most frequently diagnosed cancer and third leading cause of cancer death worldwide. In general, the incidence rate is higher in East Asia than in North America. In South Korea, newly updated data show that GC accounted for the highest cancer incidence and the fourth most frequent cause of cancer-related death in 2018(Reference Hong, Won and Lee2).
The stomach plays a vital role in the intestinal system, and it helps digest and absorb nutrients from food to the bloodstream(Reference Hornbuckle, Simpson and Tennant3,Reference Nie and Yuan4) . It also functions as a stable barrier to protect the body against harmful substances from the outside environment(Reference Groschwitz and Hogan5–Reference Vancamelbeke and Vermeire7). The risk of GC is greater among individuals in lower socio-economic classes, and environmental exposures appear to play an important part in its aetiology(Reference Crew and Neugut8). Helicobacter pylori infection is a potential risk factor for GC, especially in noncardia cancer; moreover, other contributors such as smoking, alcohol consumption, occupational exposure and diet have probable impacts on the incidence of GC(9). According to the World Cancer Research Fund and the American Institute for Cancer Research, dietary factors such as preserved/high-salt foods, grilled/barbecued or processed meat and overweight/obesity status can increase the risk of GC, while high consumption of fruit/citrus fruit and vegetables is believed to have a protective effect for GC. Limited evidence has shown the effect of riboflavin on GC(9).
Riboflavin is an essential water-soluble vitamin that is absorbed from food and is needed for the normal functional processes of cells, including growth and development(Reference Coates10–12). Furthermore, riboflavin, together with other B vitamins, serves as a cofactor for the enzyme methionine synthase reductase (MTRR) in one-carbon metabolism (OCM). Riboflavin deficiency may be implicated in the aetiology of human cancers, but the exact underlying mechanism is not clearly understood. Some observational studies have shown that riboflavin has protective effects against GC. However, other conflicting studies did not recommend using B vitamins or riboflavin for cancer prevention(Reference Dugue, Bassett and Brinkman13–Reference Xiao, Freedman and Ren16). In experimental studies, riboflavin-deficient rats showed increases in carcinogenesis, induction of DNA repair enzymes and carcinogen binding to DNA(Reference Webster, Gawde and Bhattacharya17–Reference Manthey, Rodriguez-Melendez and Hoi19). These studies have suggested that a higher intake of riboflavin especially in a deficient population would help reduce the risk of cancer.
As OCM is a key essential pathway for the processes of DNA synthesis, methylation and DNA repair, the impairment of OCM may lead to several disease outcomes and abnormal organ development(Reference Stover20). The MTRR gene is positioned on the short arm of chromosome 5, region 5p15.2–15.3(Reference Leclerc, Odievre and Wu21) and encodes for the MTRR enzyme, which is involved in the metabolic pathway of homocysteine(Reference Zeng, Liu and Tong22). At a located point, the MTRR gene can have its nucleotides replaced to make genetic polymorphisms, leading to varying genotypes. The polymorphism C524T is the substitution of a C allele with a T allele (C and T are defined as the wild-type and mutant/minor allele, respectively), which leads to a change in amino acids from serine to leucine. According to several literatures, MTRR C524T is one of the common SNP that can modify the level of the MTRR enzyme in the plasma and then interfere with its function. The results of studies investigating the effects of the MTRR C524T genetic polymorphism in the induction of cancers are also in disagreement(Reference Basir23–Reference Rouissi, Ouerhani and Oliveira32).
The genetic variants of MTRR C524T in conjunction with riboflavin insufficiency can lead to the derangement of OCM and the induction of carcinogenesis. No previous study has measured the modification effects of MTRR C524T genetic variants and riboflavin intake in GC. The main purpose of our study was to reinvestigate the relationship among riboflavin intake, a SNP of MTRR C524T and GC risk. Simultaneously, we measured the interaction between riboflavin and genetic factors in the context of incident.
Materials and methods
Subject and data collection
A case–control study recruitment for GC research project was initiated in March 2011 and finished in December 2014 at the National Cancer Center Hospital in Korea. The Institutional Review Board of National Cancer Center (NCCNCS 11-438) has already approved the study protocol.
Individuals who had been diagnosed with early-stage GC approximately 3 months before the study and who did not change their dietary pattern for any reason were included in the case group. Patients who had developed other cancers within 5 years, had an advanced stage of GC, were pregnant or breast-feeding or suffered from chronic diseases (diabetes mellitus, systemic or mental disorders) were excluded. Simultaneously, the controls were recruited from the pool of Cancer Screenee Cohort Study subjects(Reference Kim33), who visited the Center for Cancer Prevention and Detection at the National Cancer Center for a health screening. They were confirmed as not having a medical history of cancer, other stomach damage, diabetes mellitus or treatment for H. pylori.
All participants were asked to complete the surveys by themselves; the survey included questions about demographics, lifestyle, dietary habits and medical history. We categorised BMI into three ranges according to the WHO classification of weight in adult Asians: normal range (18·5–22·9 kg/m2), overweight at risk (23·0–24·9 kg/m2) and obese I–II (25–29·9 kg/m2 and ≥30 kg/m2)(34). We confirmed H. pylori infection mainly by using the rapid urease test (Pronto Dry, Medical Instruments Corp.) and assessing histology and serology. All participants were required to provide written informed consent.
Dietary intake assessment
Data on dietary food intake were collected by a semi-quantitative FFQ consisting of 106 food items(Reference Ahn, Kwon and Shim35). Information regarding the portion size and average daily frequency of food intake was recalled from the past 12 months before the study was conducted. We used CAN-PRO 4.0 (Computer Aided Nutritional Analysis Program, Korea Nutrition Society) to extract the nutritional components from the daily food intake collected by the semi-quantitative FFQ. Then, the total riboflavin intake from daily food was summed and displayed for study.
Genotype measurement
Whole-blood samples were taken from participants and then isolated to obtain peripheral blood leukocytes. After that, the genomic DNA was extracted. The Affymetrix Axiom Exome 319 Array (Affymetrix Inc.) platform, which included 318 983 variants, was used for genotyping. Genetic markers that did not fulfil the quality control criteria of a minor allele frequency < 0·05, Hardy–Weinberg equilibrium’s deviation P-value < 1 × 10–6 and low call rate (< 98 %) were rejected. The performance of the genotype imputation, an essential process for predicting unobserved genotypes in the SNP data, was assessed using the Asian population (n 504) in the 1000 Genomes Project phase III haplotypes panel from the integrated variant set release GRch37/hg19 (http://www.1000genomes.org/), which was used as a reference panel. SHAPIT (v2.r837) was used to perform phasing, and IMPUTE2 (2.3.2) was used to complete the imputation of SNP. The accuracy of those imputed genotypes was called the quality of imputation INFO score. We filtered the imputed genotypes with a stringent threshold INFO score of >0·6 to achieve the highest accuracy(Reference Marchini and Howie36–Reference Browning and Browning39). Finally, the genetic polymorphism of the MTRR gene (rs1532268) was selected.
Overall, only participants who completed the self-administered surveys and the semi-quantitative FFQ and had available data on genetic characteristics were eligible for inclusion in the final analyses. The case and control groups were then frequency-matched for sex and 5-year age distributions (2 controls per case). Finally, 756 healthy controls and 377 GC cases were selected for the analyses of the study.
Statistical analyses
We used t test for continuous variables and χ 2 test for categorical variables to compare the general characteristics of the case and control groups.
The riboflavin intake was initially adjusted for total daily energy intake using the residual method(Reference Willett and Stampfer40), and then we categorised riboflavin intake into tertile groups based on the distribution in the control group. To explore the association of riboflavin intake with GC, we computed OR with 95 % CI for each group through multiple logistic regression using the lowest group as a reference. The multivariable models were the models controlled for age, sex and energy intake (model 1) and further adjusted for first-degree family history of GC, level of education, occupation, income, smoking status and physical activity (model 2). Moreover, H. pylori was added to the fully adjusted model (model 3). A stratified analysis by H. pylori status was performed; furthermore, the median value of riboflavin intake in each tertile group was used as a continuous variable to test for trends.
The association between MTRR (rs1532268) genetic variants and GC risk was determined in the dominant model with CC as the reference group. The interaction between riboflavin intake and SNP was measured by a likelihood ratio test applying the multiplicative interaction term (SNP × riboflavin) to the multivariate logistic regression model. All analyses were performed using SAS software (version 9.4, SAS Institute).
Results
General characteristics of study participants
The general characteristics of the study participants showed some notable distinctions between the cancer and non-cancer patients (Table 1). In the cancer group, the prevalence of H. pylori-positive infection, smoking, low level of education and poor income status was higher than that in the control group. Cancer patients were more likely to have a family history of GC (20·5 %) than non-cancer patients (12·6 %). Moreover, the amount of daily riboflavin intake in mg was greater in the controls (P trend < 0·001). In the subgroups of men and women, the differences were in the same direction as in the total population analysis.
Table 1. General characteristics of the study subjects
(Mean values and standard deviations; numbers and percentages)
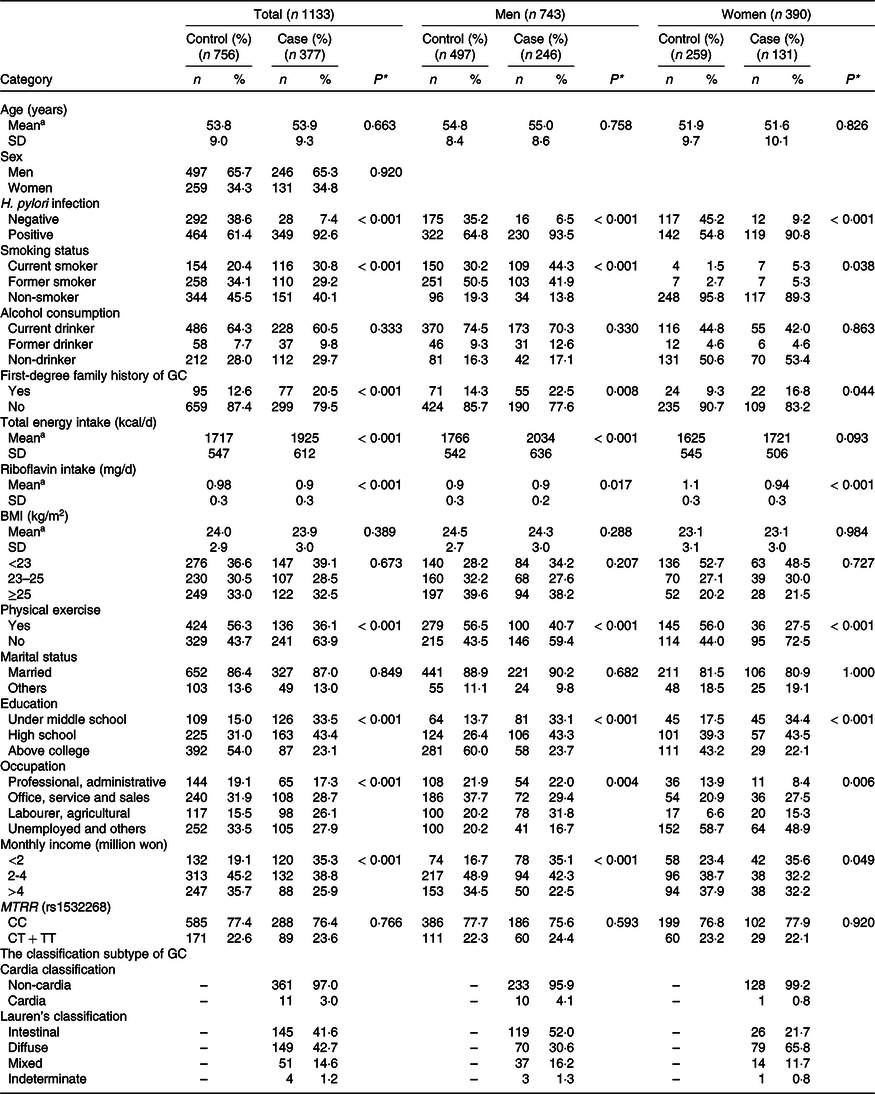
Categorical variables are summarised as the number (%) and were compared using χ 2 test.
aContinuous variables are summarised as the mean and standard deviation (SD) and were compared using t test.
*P values denote the difference between the cases and controls at the 95 % confidence level. Significant P value < 0.05.
Association between riboflavin intake and gastric cancer risk
The tertile ranges of riboflavin intake were significantly associated with GC risk in the total population of 1133 individuals and in the subgroup of 390 women (Table 2). Compared with the lowest tertile, the highest tertile exhibited a remarkable reduction in the risk of GC by approximately 54 % in the general population (T1 as reference) (OR = 0·46, 95 % CI = 0·33, 0·63, P trend < 0·001), even after adjusting for age, sex, total energy intake, first-degree family history of GC, occupation, level of education, smoking status, income and physical activity (OR = 0·57, 95 % CI = 0·40, 0·82, P trend = 0·002) as well as H. pylori infection status (OR = 0·56, 95 % CI = 0·39, 0·81, P trend = 0·002). Further analyses in the sex subgroups showed significant results only in females in the three aforementioned adjusted models (OR = 0·39, 95 % CI = 0·23, 0·67; OR = 0·54, 95 % CI = 0·30, 0·98; OR = 0·52, 95 % CI = 0·28, 0·97, respectively, all P trend < 0·05). As shown in Table 3, the results were in the same direction in the H. pylori infection subgroups after controlling for potential confounding factors. A higher intake of riboflavin was related to a reduction in GC risk in both the positive and negative infection groups (OR = 0·60, 95 % CI = 0·40, 0·89, P trend = 0·009; OR = 0·20, 95 % CI = 0·05, 0·78, P trend = 0·019, respectively).
Table 2. Association between riboflavin intake stratified by tertiles and gastric cancer risk
(Numbers and percentages; odds ratio and 95 % confidence intervals)
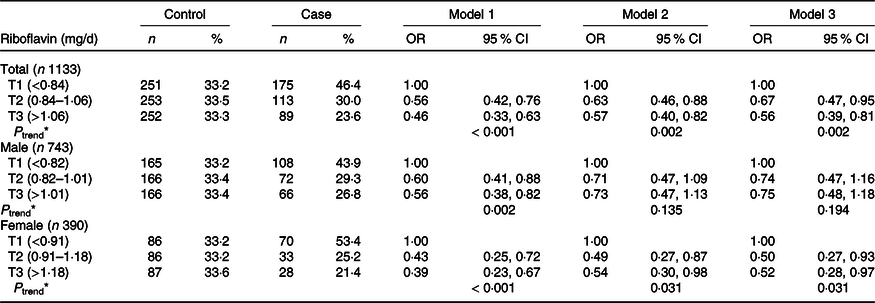
Riboflavin was categorised into tertiles (T1, T2, T3) based on the distribution of the control group. Multiple logistic regression for the association, using the lowest group as a reference. Model 1: adjusted by age, sex, energy intake. Model 2: adjusted by age, sex, energy intake, first-degree family history of gastric cancer, education level, job, household income, smoking status, regular exercise. Model 3: additional adjustment for H. pylori infection status. *P-trends < 0·05 denote significant level.
Table 3. Association between riboflavin intake by tertiles and gastric cancer risk, stratified by H. pylori infection status
(Odds ratio and 95 % confidence intervals)
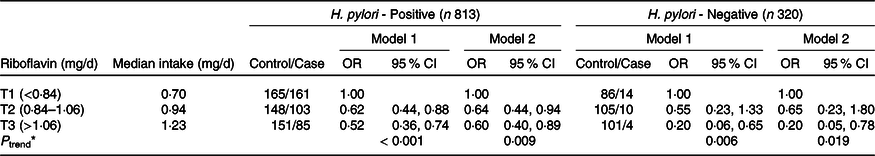
Riboflavin was categorised into tertiles (T1, T2, T3) based on the distribution of control group. Multiple logistic regression for the association, using the lowest group as a reference. Model 1: adjusted by age, sex, energy intake. Model 2: adjusted by age, sex, energy intake, first-degree family history of gastric cancer, education level, job, household-income, smoking status, regular exercise. *P-trends < 0·05 denote significant level.
Association between the MTRR (rs1532268) C524T genetic polymorphism and gastric cancer risk
As shown in Table 4, MTRR C524T was individually analysed in the dominant model (CC v. TC + TT genotypes). A significant association was not observed between the MTRR C524T genetic polymorphism and the risk of GC. However, when we investigated the interaction of the MTRR gene at the C524T SNP and riboflavin intake in GC risk, MTRR C524T and riboflavin intake was found to have synergistic carcinogenic effects (Table 5). Compared with carriers of the CC genotype, low riboflavin intake in T+ carriers was significantly associated with a 93 % higher risk of GC (OR = 1·93, 95 % CI 1·09, 3·42, P for interaction = 0·037). In general, higher riboflavin intake may help reduce the risk of GC in both CC and TC + TT carriers, particularly in T+ carriers with borderline significance (OR = 0·54, 95 % CI 0·28, 1·02, P for interaction = 0·037).
Table 4. Associations of MTRR (rs1532268) genetic polymorphisms and gastric cancer risk in the dominant model
(Numbers and percentages)
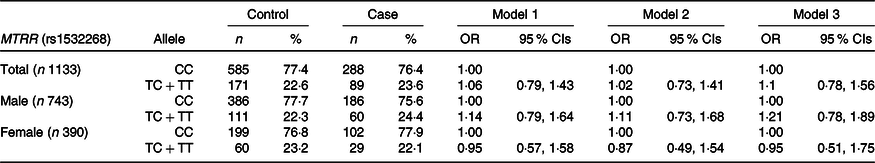
Model 1: adjusted by age, sex, energy intake. Model 2: adjusted by age, sex, energy intake, first-degree family history of gastric cancer, education level, job, household-income, smoking status, regular exercise. Model 3: additional adjustment for H. pylori infection status.
Table 5. Interaction between MTRR (rs1532268) genetic polymorphisms and riboflavin intake in gastric cancer risk
(Numbers and percentages)
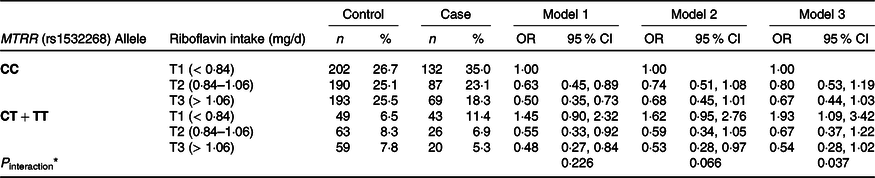
Riboflavin was categorised into tertiles (T1, T2, T3) based on the distribution of control group. Multiple logistic regression for the association, using the lowest riboflavin level in CC group as a reference. Model 1: adjusted by age, sex, energy intake. Model 2: adjusted by age, sex, energy intake, first-degree family history of gastric cancer, education level, job, household income, smoking status, regular exercise. Model 3: additional adjustment for H. pylori infection status. *P interaction is the P trend of the interaction between riboflavin intake and MTRR genotype.
Discussion
In our study, which comprised 377 cases and 756 controls, we observed a protective impact of daily riboflavin intake against GC and the genetic variants of MTRR C524T also played a role in this association. At MTRR C524T, the T allele was considered to be a risk allele because, among individuals with the same low level of riboflavin intake, T+ carriers tended to have a higher risk of GC than CC carriers. In other words, high riboflavin consumption significantly decreased the risk of GC in the TC and TT carriers and this decrease in risk was more apparent than in the CC carriers.
A cohort study including 323 GC patients (n 73 009) in 2014 explored the significant protective association of riboflavin against GC in premenopausal women(Reference Kweon, Shu and Xiang15). In 1993, a randomised controlled trial with 29 584 participants conducted over 5 years of follow-up identified the anticancer effect of some nutritional supplements, including combined riboflavin–niacin tablets(Reference Blot, Li and Taylor41). Moreover, two case–control studies displayed the same correlation direction as our studies, with sample sizes of 272 and 2575(Reference Ji, Chow and Yang42,Reference Kim, Kim and Chang43) . However, contrary results were revealed in other studies with limited study populations ranging from 318 to 777, in which riboflavin intake had no association with the risk of GC(Reference Dugue, Bassett and Brinkman13,Reference Chen, Tucker and Graubard44,Reference Pelucchi, Tramacere and Bertuccio45) , or it could somehow increase GC risk in varied study sample sizes ranging from 492 to 3152 subjects(Reference Risch, Jain and Choi46,Reference Kaaks, Tuyns and Haelterman47) . The inconsistency could be explained by the limited sample sizes in almost all previous case–control studies and the differences in dietary pattern collection and measurement methods. Importantly, the differences could also be due to the difference in regional distribution. All of the results that were in agreement with our studies were conducted on Asian populations (China, Korea)(Reference Kweon, Shu and Xiang15,Reference Blot, Li and Taylor41–Reference Kim, Kim and Chang43) , while the others were in Belgium, Australia, northern Italy, Canada and the USA(Reference Dugue, Bassett and Brinkman13,Reference Chen, Tucker and Graubard44–Reference Kaaks, Tuyns and Haelterman47) , where distinct gaps with the Asian countries in terms of dietary patterns/habits, culture, genes and other potential characteristics exist. In addition to riboflavin consumed in food, riboflavin supplements might contribute to the overall protective effect of riboflavin. Hence, the inconsistent results in the above studies could be driven by the difference in vitamin supplement habits between individuals or populations, which was not assessed in this study. One possible hypothesis regarding the increase in GC risk is that milk is rich in riboflavin, and people who have preceding cancer symptoms tend to drink more milk to relieve discomfort. While milk/dairy product consumption is positively associated with GC in some studies(Reference Wang, Zhou and Ji48), this hypothesis indicates the inability to eliminate the possibility of a spurious association (harmful effect of riboflavin) based on a case–control study design. The results of this study showed that riboflavin intake was only negatively associated with GC risk in the female subgroup, whereas no significant association was found in the male subgroup. A reasonable explanation for this difference is that oestrogen exposure in females is protectively associated with GC risk as found in previous studies(Reference Camargo, Goto and Zabaleta49,Reference Frise, Kreiger and Gallinger50) . Moreover, the oestrogen hormone and its receptors, which are present in gastric tissue, were shown to interfere with GC development and prognosis in some studies(Reference Ryu, Kim and Jang51,Reference Tang, Liu and Yan52) . However, the difference in sample size, average energy intake and mean riboflavin consumption between the two subgroups, which could not be eliminated in our study, could have led the existence of a spurious association.
Some experimental studies strongly supported the carcinogenic effect of insufficient riboflavin due to an increase in protein and DNA oxidative damage(Reference Webster, Gawde and Bhattacharya17,Reference Manthey, Rodriguez-Melendez and Hoi19) , an increase in carcinogen binding to DNA(Reference Pangrekar, Krishnaswamy and Jagadeesan18) and the over-induction of DNA repair enzymes(Reference Webster, Gawde and Bhattacharya17). The anticancer mechanism of riboflavin is unclear, but the effect may be related to its antioxidant effects and strong involvement in OCM. The antioxidant properties of riboflavin are mainly demonstrated by its role in the glutathione redox cycle, which therefore can lead to an increase in the dysfunction of the antioxidant-glutathione in some circumstances. Moreover, riboflavin also affected the activity of catalase, superoxide dismutase enzymes and some other antioxidant enzymes. Hence, insufficient consumption of riboflavin and the resulting reduction in enzyme activities would impair the antioxidant defence system of the body and lead to an imbalance in the oxidant–antioxidant system(Reference Saedisomeolia and Ashoori53–Reference Rivlin55). As an important cofactor in OCM, which is a critical network of several biological processes, such as DNA synthesis, methylation and DNA repair, riboflavin deficiency in humans may have consequences for DNA single-strand breaks and anomalous DNA repair(Reference Webster, Gawde and Bhattacharya17,Reference Huang and Vieira56) , which contribute to the induction of carcinogenesis. Another mechanism identified in experimental studies is that riboflavin helps to maintain epithelial integrity. Hence, the lack of riboflavin could lead to a higher risk of epithelial dysplasia in the upper gastrointestinal tract(Reference Wynder and Klein57,Reference Wynder and Chan58) .
In our study, MTRR C524T showed no significant association with GC, which is in agreement with two other studies conducted in 2012 and 2014(Reference Chang, Chang and Butler26,Reference Yoo, Kim and Hwang27) . In 2007, a study in Poland showed that the T allele in Ex5 + 123C > T (another name for C524T) marginally increased the risk of GC (OR = 1·30; 95 % CI 0·93, 1·82)(Reference Zhang, Terry and Hou25). Regarding other cancer types, the T allele in MTRR C524T was a harmful factor for prostate and breast cancer(Reference Basir23,Reference Lissowska, Gaudet and Brinton24) , while no association was found for oesophageal, colorectal, liver, lung or bladder cancer(Reference Chang, Chang and Butler26,Reference Pardini, Kumar and Naccarati28–Reference Rouissi, Ouerhani and Oliveira32) . As no other study exists on MTRR C524T and GC, hypothesising about the reason for this discrepancy is challenging, with the exception of noting the regional characteristics, study designs and small sample sizes. Although an association between the rs1532268 SNP and GC was not observed in our study, a significant interaction was found between the candidate SNP and riboflavin intake that modifies the association between riboflavin and GC risk. Because the MTRR enzyme along with riboflavin is the crucial component in the OCM pathway, the impact of MTRR gene variants on the anticancer capacity of riboflavin is reasonable and explainable.
Because our research used a case–control design, several limitations still exist. We could not eliminate the possibility of selection bias and recall bias. Because this study had a hospital-based design, the controls were healthy people participating in the health screening who tended to have a higher awareness of illnesses and may have healthier lifestyles/habits; thus, they might not be representative of the general population. Additionally, the duration of riboflavin intake is very important: the long-term use of combined riboflavin tablet was found to result in a more substantial reduction in the cancer risk in a randomised controlled trial(Reference Blot, Li and Taylor41); however, we could not clearly define the exposure time of our factor of interest. Additionally, as mentioned above, riboflavin supplements might also have contributed to the favourable effect of overall riboflavin, but this study did not assess this potential variable. However, the current study proposed to assess dietary riboflavin intake, not supplemental intake, and the evidence regarding the association of riboflavin supplements and GC is quite limited. Moreover, recall bias could have occurred due to the long period of dietary intake reported by participants (12 months). Temporal bias is also acknowledged because the dietary food assessment was performed after the disease diagnosis was confirmed. Certainly, even with our best recruitment effort, the sample size was relatively small to allow for sufficient statistical power.
Although there are unavoidable limitations, our study has some strengths that can inform future projects. (1) The current study is the first to address the interaction of MTRR rs1532268 variants with riboflavin intake in the context of GC risk. (2) We used a comprehensive and validated semi-quantitative FFQ with 106 items for dietary data collection and riboflavin intake calculation. (3) Additionally, the information of the case group was collected by well-trained interviewers using a self-reported method with strict rechecking of missing data in the control group. (4) Finally, almost all information about potential risk factors for GC, such as H. pylori infection, smoking and alcohol consumption, was completely available. Hence, the study had more advantages with regard to controlling for all possible confounders.
Conclusion
According to our research, riboflavin is a protective factor for GC. At MTRR C524T, the T allele was considered to be a risk allele for GC in the Korean population. Our study offers a hopeful direction for clinical practice in the future by demonstrating that T allele carriers should be encouraged to consume more riboflavin for better GC prevention. However, a larger sample size and better validity design are needed for the upcoming studies to reconfirm this interaction.
Acknowledgements
This work was supported by grants from the International Cooperation & Education Program (NCCRI•NCCI 52210-52211, 2020) of the National Cancer Center, the National Cancer Center (191033) and the National Research Foundation of Korea (2021R1A2C2008439).
Formal analysis: Y.-T. L., M. G. and J. L.; preparation of original draft: Y-T. L.; writing-review and editing: M. G. and J. K.; data curation, I. J. C., Y.-I. K. and J. K.; investigation: I. J. C. and Y.-I. K.; methodology: I. J. C., Y.-I. K. and J. K.; funding acquisition: J. K.; project administration: J. K. and supervision: J. K.
All the authors declare no conflicts of interest.








