It is well known that proteins elicit greater thermic effects, increase resting energy expenditure (REE)( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 1 – Reference Halton and Hu 3 ) and satiety( Reference Apolzan, Carnell and Mattes 4 , Reference Poppitt, Proctor and McGill 5 ) and decrease respiratory exchange ratios in the immediate postprandial period( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 1 , Reference Hursel, van der Zee and Westerterp-Plantenga 6 , Reference Alfenas Rde, Bressan and Paiva 7 ) when compared with carbohydrates (CHO) and fats. Furthermore, the addition of a protein supplement to a normal diet promotes weight loss and maintenance, mainly as a result of increases in satiety( Reference Latner and Schwartz 8 , Reference Soenen and Westerterp-Plantenga 9 ) and energy expenditure( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 1 , Reference Westerterp, Wilson and Rolland 10 , Reference Westerterp-Plantenga, Rolland and Wilson 11 ).
The positive physiological effects of protein consumption have primarily been observed in studies investigating the postprandial effects of mixed meals consumed in the morning and afternoon( Reference Westerterp-Plantenga, Rolland and Wilson 11 – Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 14 ) or after consumption of single macronutrients in the hours close to exercise( Reference Andersen, Tufekovic and Zebis 15 – Reference Res, Groen and Pennings 17 ). However, less is known about the effects of different macronutrients consumed in the late evening within 30 to 60 min of sleep on the desire to eat and REE the following morning.
Night-time eating is a largely unexplored component of nutrient timing studies and could be a key factor that plays an instrumental role in the modulation of the effects of different macronutrients on body composition, metabolism and satiety. Until recently, the majority of the information regarding what individuals should be consuming at night has been anecdotal and based on the idea that late-evening ingestion of food will increase the likelihood of weight gain( Reference de Castro 18 ). The potential for weight gain with late-evening food consumption is plausible given that both metabolic rate( Reference Katayose, Tasaki and Ogata 19 ) and satiety( Reference de Castro 18 ) are reduced during this time, which may favour a positive energy balance. For this reason, it has been recommended that individuals concerned with weight regulation avoid consuming energy-containing products in the hours close to sleep due to the potential body composition implications associated with increased food intake and attenuated physiological functioning. However, this is not the case for physically active individuals who would probably benefit from a constant flow of nutrients that may be necessary for optimal recovery both post-exercise and during the overnight period( Reference Res, Groen and Pennings 17 , Reference Beelen, Tieland and Gijsen 20 ).
Interestingly, the milk proteins, whey protein (WP) and casein protein (CP), are digested and absorbed at different rates( Reference Boirie, Dangin and Gachon 21 ). CP has been described as a ‘slow protein’ due to the slower digestion and absorptive rates observed compared with those of WP, and therefore CP has popularly been recommended as a night-time protein( Reference Boirie, Dangin and Gachon 21 ). Furthermore, in some( Reference Hursel, van der Zee and Westerterp-Plantenga 6 , Reference Alfenas Rde, Bressan and Paiva 7 ), but not all( Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 14 ), studies, CP has been shown to increase satiety and reduce appetite to a greater extent when compared with WP. Recently, Res et al. ( Reference Res, Groen and Pennings 17 ) have reported that oral CP consumption before sleep results in elevated muscle protein synthesis throughout the sleeping hours compared with non-energy-containing placebo (PLA) consumption in healthy young men after a resistance exercise bout. Another study carried out in this laboratory has demonstrated that CP administered via nasogastric tubing during sleep increased overnight muscle protein synthesis in elderly men( Reference Groen, Res and Pennings 22 ). These data highlight the potential benefits of providing a constant flow of nutrients through night-time protein intake. It is possible that night-time protein intake may be a new window of opportunity for physiological benefits and, in the long term, optimal performance, but no studies have compared the effects of different proteins consumed before bed.
Therefore, the present study investigated the extent to which a single serving of WP, CP, CHO or a PLA before sleep affects satiety and metabolism, independent of exercise, in healthy, physically active young men. We hypothesised that consumption of protein at night before sleep would have a positive impact on next-morning metabolism and appetite to a greater extent than that of CHO or a PLA beverage.
Methods
Participants
In the present study, eleven physically active ( ≥ 4 d/week and 50 min/d of self-reported moderate-to-vigorous physical activity for >12 months) college-aged men (age: 23·6 (sem 1·0) years; height: 183·1 (sem 2·2) cm; weight: 86·2 (sem 3·5) kg; BMI: 25·8 (sem 0·8) kg/m2; and body fat: 16·3 (sem 2·5) %) participated. Participants were excluded if they had uncontrolled hypertension (blood pressure >160/100 mmHg), were taking blood pressure or cholesterol medications, or had been diagnosed with CVD, stroke, diabetes, or thyroid or kidney dysfunction or had milk allergies. Additionally, all smokers were excluded. The participants were asked to refrain from taking any nutritional supplements (except for a multivitamin) for 2 weeks before their first laboratory visit and throughout the duration of the study. In addition, the participants were asked to maintain their usual exercise regimen for the duration of the study. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Florida State University Institutional Review Board. Written informed consent was obtained before participation in the study.
Study design
The study had a randomised, double-blind, cross-over design. Before the start of the study, the participants reported to the laboratory for baseline measurements of height (Seca Corporation), weight (Detecto®; DETECTO Scale Company), waist and hip circumferences (Creative Health Products, Inc.), and body composition via air-displacement plethysmography (BOD POD®; COSMED). WP and CP are the commonly consumed supplements by physically active individuals and have been associated with improvements in many physiological outcomes( Reference Res, Groen and Pennings 17 , Reference Groen, Res and Pennings 22 – Reference Hall, Millward and Long 24 ). Therefore, these proteins were administered in the present study. The following four supplements were randomly consumed with each trial being separated by 48–72 h: (1) WP (38 g, 628 kJ (150 kcal), 30 g protein, 3 g CHO and 2 g fat); (2) CP (38 g, 586 kJ (140 kcal), 30 g protein, 3 g CHO and 1 g fat); (3) CHO (maltodextrin; 38 g, 628 kJ (150 kcal), 0 g protein, 33 g CHO and 2 g fat); (4) PLA (2·9 g, 0 kJ (0 kcal); Propel Zero™; PepsiCo Inc.). Powdered WP, CP and CHO were labelled A, B and C and packaged by an external investigator who was not otherwise involved in the study. All powders were flavoured identically (vanilla chai) and had an identical texture to ensure that the participants were truly blinded to each experimental trial. The non-energy-containing PLA (Propel Zero™) was also in a powdered form and was labelled as supplement D. The flavour and consistency of the PLA were different from those of the WP, CP and CHO powders. Therefore, the PLA was single blinded and not double blinded to the participants. The administered dose of 30 g was chosen because it has previously been shown to be ideal for healthy, physically active men( Reference Symons, Sheffield-Moore and Wolfe 25 , Reference Moore, Robinson and Fry 26 ), and the 627 kJ (150 kcal) serving was chosen in an attempt to offer a reasonable energetic load that mimics many manufactured protein beverages. All supplements were consumed at home with 12 oz of water as the last food or energy-containing beverage of the day, taken at least 1 h following consumption of the participant's last evening meal and within 30 min of going to sleep. The morning following the evening consumption (05.00–08.00 hours), the participants reported to the laboratory for the measurement of satiety and metabolism.
On two consecutive days before each experimental trial, all the participants completed a dietary log and were asked to replicate this eating pattern before each visit. Compliance was verified by the analysis of food records by the same research technician (United States Department of Agriculture, www.choosemyplate.gov). Furthermore, the participants were instructed to refrain from consuming caffeine or alcohol and undertaking any planned physical activity 24 h before each visit.
Hunger, satiety and desire to eat assessment
Upon arrival to the laboratory on each test morning, the participants completed a visual analogue scale( Reference Flint, Raben and Blundell 27 ) to subjectively assess hunger, satiety and desire to eat. The visual analogue scale is a 100 mm horizontal scale with opposing extremes (‘not at all’ to ‘extremely’) of each appetite sensation (hunger, satiety and desire to eat) anchored at each end of the 100 mm line. The participants indicated their subjective feelings by placing a vertical line along the 100 mm scale, and each rating was converted to a score in mm using a standard ruler. Higher scores indicated greater feelings of each sensation.
Resting energy expenditure
Following the visual analogue scale measurements, the participants were asked to lie supine on a bed in a dark, quiet and climate-controlled room (20–22°C) for 30 min in an effort to have them feel completely rested. Gas exchange was then measured continuously for 60 min to assess VO2 (ml/kg per min), REE (kcal/d) and respiratory quotient via indirect calorimetry using a ventilated hood (TrueOne 2400 metabolic cart; ParvoMedics). The last 50 min of the data collection period were used for analysis and compared across five 10 min segments over the last 50 min period. The test–retest intra-class CV for the measurement of REE in our laboratory is 1·7 %.
Anthropometrics and body composition
Upon completion of the metabolic measurements, the participants had their height and weight measured without shoes while wearing only spandex shorts to the nearest 0·1 cm and 0·1 kg, respectively, via a wall-mounted stadiometer (Seca Corporation) and a digital scale (Detecto®). Waist and hip circumferences were taken a minimum of two times using a Gulick fibreglass measuring tape with a tension handle (Creative Health Products, Inc.). Additional measurements were taken if duplicate readings were in excess of 5 mm of each other, until the discrepancy between the two readings was equal to or less than 5 mm. Body composition (% body fat, lean and fat mass) was measured with the participants wearing spandex shorts and a lycra cap over their hair via air-displacement plethysmography (BOD POD®; COSMED)( Reference McCrory, Gomez and Bernauer 28 ).
Statistical analyses
Statistical analyses were conducted using JMP Pro 10 (SAS). A 4 × 5 (group × time) repeated-measures ANOVA was conducted to measure differences during each of the five 10 min segments of the final 50 min period of indirect calorimetry. A one-way ANOVA was conducted for the measurement of hunger, satiety and desire to eat for each trial. Tukey's post hoc analysis was used where appropriate to examine group differences. A Shapiro test was used to ensure normality for body fat percentage and BMI. Significance was set at P <0·05, and data are reported as mean with their standard errors, unless otherwise noted.
Results
There were no differences in total energy, protein, CHO or fat consumed before any of the four experimental trials. The analysis of the 2 d dietary logs indicated that an average of 9223 (sem 3169) kJ/d (2204 (sem 757) kcal/d) was consumed (19·9 (sem 6·6) % protein, 48·4 (sem 9·5) % CHO and 30·8 (sem 8·2) % fat).
Subjective assessments of hunger, satiety and desire to eat are presented in Table 1. No significant differences were observed for hunger, satiety or desire to eat among the WP, CP, CHO and PLA groups. However, although not statistically significant, satiety (feeling of fullness) after consumption of both WP (40·6 (sem 5·4) mm) and CP (45·4 (sem 0·4) mm) was greater than that after consumption of CHO (36·1 (sem 5·4) mm) and PLA (33·9 (sem 5·4) mm). Metabolic assessment of VO2 (ml/kg per min) was done continuously for 60 min, and 10 min mean intervals from the final 50 min period were analysed (Fig. 1). There were no group × time interactions or time effects; however, there were group differences. Mean VO2 (ml/kg per min) for the WP (3·35 (sem 0·03) ml/kg per min) and CP (3·30 (sem 0·03) ml/kg per min) groups were significantly (P <0·0001) greater than those for the PLA group (3·16 (sem 0·03) ml/kg per min) but not for the CHO group (3·25 (sem 0·03) ml/kg per min) (Fig. 1(a)). Additionally, there were no significant differences between the WP and CP groups or between the CHO and PLA groups for VO2 (Fig. 1). Interestingly, although not statistically significant, the PLA group had a lower VO2 at 20, 30, 40 and 50 min than the WP, CP and CHO groups (Fig. 1(b)).
Table 1 Visual analogue scale of hunger, satiety and desire to eat (n 11) (Mean values with their standard errors)
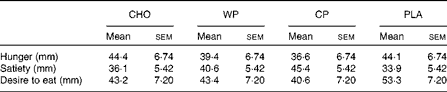
CHO, carbohydrate; WP, whey protein; CP, casein protein; PLA, placebo.
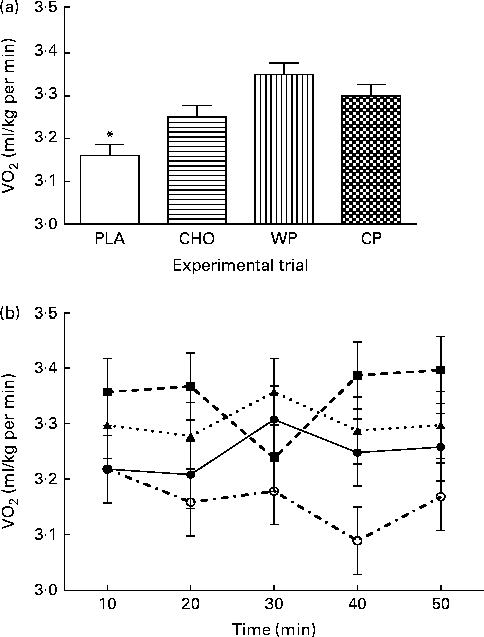
Fig. 1 (a) VO2 (ml/kg per min) and (b) 10 min intervals of VO2 (ml/kg per min) for 50 min the morning after night-time consumption of a single serving of whey protein (WP), casein protein (CP), maltodextrin (CHO) and a non-energetic placebo (PLA). Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of WP and CP (P< 0·05). (b) ![]() , PLA;
, PLA; ![]() , CHO;
, CHO; ![]() , WP;
, WP; ![]() , CP.
, CP.
The predicted REE (kJ/d; kcal/d) measurements during 10 min mean intervals from the final 50 min of the data collection period are presented in Fig. 2. There were no group × time interactions or time effects; however, there were group differences. The predicted REE was significantly greater after consumption of the WP (8151 (sem 65) kJ/d; 1947 (sem 16) kcal/d), CP (8126 (sem 65) kJ/d; 1941 (sem 16) kcal/d) and CHO (7988 (sem 65) kJ/d; 1908 (sem 16) kcal/d) than after that of the PLA (7716 (sem 65) kJ/d; 1843 (sem 16) kcal/d; P <0·0001). There were no significant differences between the WP, CP and CHO groups. Similar to that observed for VO2, there were no differences at baseline (minute 10) and, although not significant, the PLA group had the lowest REE at all the other time points. Respiratory quotient (VCO2/VO2) was significantly lower after consumption of the PLA (0·76 (sem 0·003)) than after that of the WP (0·77 (sem 0·003)) and CHO (0·77 (sem 0·003); P <0·0001), but not after that of the CP (0·76 (sem 0·003)) (Fig. 3).
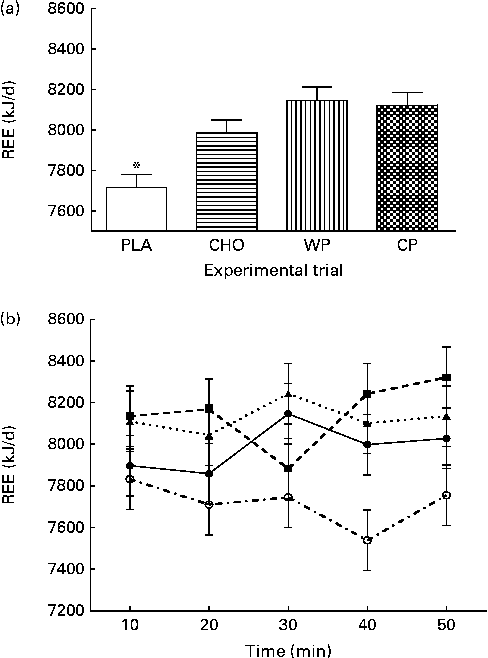
Fig. 2 (a) Predicted resting energy expenditure (REE, kJ/d) and (b) 10 min intervals of predicted REE (kJ/d) for 50 min the morning after night-time consumption of a single serving of whey protein (WP), casein protein (CP), maltodextrin (CHO) and a non-energetic placebo (PLA). Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of WP, CP and CHO (P< 0·05). (b) ![]() , PLA;
, PLA; ![]() , CHO;
, CHO; ![]() , WP;
, WP; ![]() , CP.
, CP.
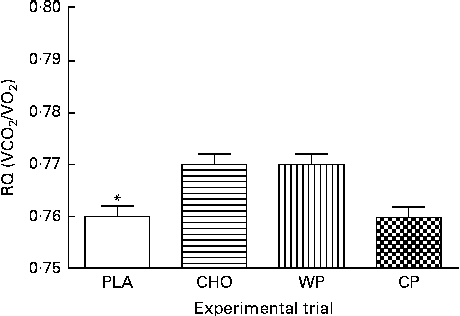
Fig. 3 Respiratory quotient (RQ) the morning after night-time consumption of a single serving of whey protein (WP), casein protein (CP), maltodextrin (CHO) and a non-energetic placebo (PLA). Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of WP and CHO (P< 0·05), but not CP.
Discussion
The ability of proteins to increase thermogenesis, and therefore REE, to a greater extent than CHO has been well documented in acute studies( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 1 , Reference Hursel, van der Zee and Westerterp-Plantenga 6 , Reference Karst, Steiniger and Noack 29 – Reference Nair, Halliday and Garrow 31 ). The positive physiological effects of proteins have been observed after daytime consumption( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 1 , Reference Alfenas Rde, Bressan and Paiva 7 , Reference Lorenzen, Frederiksen and Hoppe 32 ) or in the hours close to resistance exercise( Reference Andersen, Tufekovic and Zebis 15 , Reference Res, Groen and Pennings 17 , Reference Benton and Swan 33 ). However, to our knowledge, no studies have investigated satiety or REE changes after protein consumption before sleep, and none has compared then after WP, CP and CHO consumption during this time frame.
The main findings of the present study are that there were no differences between the effects of protein and CHO on morning REE or subjective feelings of satiety when consumed before sleep in healthy, physically active young men. Furthermore, there were no significant differences between the effects of WP and CP consumption on morning REE. Interestingly, the WP, CP and CHO elicited a significantly greater effect on morning REE than the PLA. Our data suggest that, regardless of the macronutrient type, consuming 586–628 kJ (140–150 kcal) of a supplement in liquid form before bed has a superior effect on morning VO2 and REE than going to bed on an empty stomach. This finding has relevance to healthy, physically active individuals and may extend to populations that are trying to lose and/or maintain body weight. Traditional practice has been to limit energy intake in the late evening in an attempt to prevent storing energy consumed as fat because metabolism is thought to slow during sleep. Although metabolism has been shown to slow at night( Reference Katayose, Tasaki and Ogata 19 ), no scientific evidence has shown this to be true after consuming food before sleep. Our findings suggest that energy intake of 586–628 kJ (140–150 kcal) before sleep actually increases VO2 and predicted REE.
It is well documented that proteins have a superior thermogenic and satiating effect than CHO and fats, and still less is known whether differences exist between protein types. In agreement with the present results, Lorenzen et al. ( Reference Lorenzen, Frederiksen and Hoppe 32 ) reported no differences in postprandial REE between three 1465 kJ (350 kcal) mixed breakfast meals, each containing 36 g WP, 34 g CP or milk (28 g casein and 7 g whey). In contrast, Acheson et al. ( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 1 ) found the thermic effect of 1921 kJ (459 kcal) of a WP mixed meal to be greater than that of isoenergetic casein and soya protein meals and found all protein groups to have a greater thermogenic effect than 1921 kJ (459 kcal) of CHO in twenty-three healthy lean men and women. Moreover, 30 g of WP (502 kJ or 120 kcal) did not affect REE in middle-aged women and decreased fat oxidation compared with a non-energetic placebo when consumed immediately post-exercise( Reference Benton and Swan 33 ). These conflicting findings demonstrate the need for more research investigating the effect of protein choice, particularly at night, on metabolism. Furthermore, the energy consumed in the experimental trials of the aforementioned studies varied and were different from the 586–628 kJ (140–150 kcal) used in the present study. Therefore, future research should focus on determining the optimum energetic load needed to elicit favourable changes in REE. Nevertheless, consumption of 40 g of CP before sleep has been shown to stimulate muscle protein synthesis overnight in both young( Reference Res, Groen and Pennings 17 ) and elderly( Reference Groen, Res and Pennings 22 ) men, and it is likely that, in the long term, this increase in muscle protein synthesis will result in an increase in REE. The energy load reported by these studies is at least 669 kJ (160 kcal), which is very similar to the energy load observed in the present study. However, these authors did not specify whether any additional energy was provided by their casein supplement, so a true comparison is not possible.
When compared with CHO, several studies have observed an increased satiating effect of protein-rich breakfast and lunch in the postprandial period( Reference Halton and Hu 3 , Reference Latner and Schwartz 8 , Reference Paddon-Jones, Westman and Mattes 34 , Reference Bowen, Noakes and Trenerry 35 ). Although not significant, the present results indicate that a single serving of WP or CP before sleep has a greater satiating effect than that of CHO. The present results are in agreement with those reported by Bowen et al. ( Reference Bowen, Noakes and Trenerry 35 ), who found proteins to improve satiety more than CHO. Although the improved satiating effect of proteins compared with that of CHO has been demonstrated, the debate regarding which type of protein best modulates satiety is ongoing. The satiating effects of certain proteins may be dose dependent, as shown by Smeets et al. ( Reference Smeets, Soenen and Luscombe-Marsh 36 ), who reported energy expenditure and satiety to be acutely improved after consumption of a single high-protein lunch (25 % energy from protein) than after that of an adequate protein meal (10 % energy from protein) in thirty healthy men and women (P <0·02) with each meal comprising 35 % of each participant's daily energy requirements. More recently, Veldhorst et al. ( Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 12 – Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 14 ) have examined the effects of a mixed breakfast meal containing whey, casein or soya protein at fixed doses (20 % of daily energy requirement) on satiety for 4 h after ingestion in thirty healthy men and women. A breakfast containing 10 % energy from whey was more satiating than that containing 10 % casein at all time points and 10 % soya 20 min after breakfast (P <0·05)( Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 14 ). There were no differences between proteins at 25 % energy intake, an intake considered higher than normal, suggesting that after a certain amino acid threshold, whey, casein and soya as part of a breakfast have similar satiating effects( Reference Veldhorst, Nieuwenhuizen and Hochstenbach-Waelen 14 ). The dose-dependent effects of different proteins on subjective assessments of satiety may explain the similarities between the WP and CP treatments in the present study, as both used 30 g of the respective proteins.
There are a number of potential reasons for the discrepancy between the findings of the aforementioned studies and our findings. To date, the effects on satiety have been reported after daytime protein consumption and typically as part of a mixed meal. The present study, however, investigated the effects of a single serving of different proteins consumed before sleep on morning (8–10 h later) satiety. Furthermore, we measured subjective feelings of hunger, satiety and desire to eat several hours after consumption of the WP, CP, CHO or PLA. Thus, the elapsed time may have contributed to the lack of significant differences in the measures of satiety. In addition, it has also been reported that individuals with habitually high protein intakes have a diminished satiating response to a single protein meal( Reference Long, Jeffcoat and Millward 37 ). As our participants were active college-aged men, their diets may have contained higher-than-standard protein amounts (1·3 g/kg per d; 19·8 (sem 5·3) % protein), which may explain the lack of a significant effect on appetite in the present study. Furthermore, Groen et al. ( Reference Groen, Res and Pennings 22 ) reported that overnight administration of 40 g of casein elicited normal dietary protein digestion and absorption kinetics. In addition, during the 5 h overnight postprandial period, the authors observed that the amount of available circulating protein from the casein bolus was similar to that observed after whey consumption during the day. In addition, other authors have reported similar amino acid availability 5 h following morning whey or casein consumption( Reference Boirie, Gachon and Corny 38 – Reference Pennings, Koopman and Beelen 40 ). These findings suggest similar protein digestion and absorption kinetics overnight between the WP and CP, thereby possibly explaining our lack of significant differences between protein types.
Further inconsistency exists in the literature with regard to macronutrient type and fat oxidation. Similar to our findings, Benton et al. ( Reference Benton and Swan 33 ) reported fat oxidation (respiratory exchange ratios) to be significantly diminished after 30 g post-exercise whey supplementation (502 kJ or 120 kcal) 120 min after consumption compared with PLA supplementation (P= 0·02). We found fat oxidation, as assessed by respiratory exchange ratios, to be significantly lower after consumption of the WP and CHO (P= 0·0003) but not after that of the CP. WP has been shown to have significantly (P <0·05) lower( Reference Acheson, Blondel-Lubrano and Oguey-Araymon 1 ) and higher( Reference Lorenzen, Frederiksen and Hoppe 32 ) respiratory exchange ratio values than CHO and CP, respectively. Interestingly, in the present study, in addition to having a significantly greater REE and VO2 response, the CP group had a response similar to that of the PLA group for fat oxidation. While we were unable to collect blood in the present study, Pennings et al. ( Reference Pennings, Boirie and Senden 23 ) reported that casein ingestion results in a significantly lower insulin response when compared with WP or CHO ingestion. Insulin can dramatically lower fat oxidation, and this could explain the small differences in fat oxidation that we observed. The slow-digesting nature of CP may have resulted in a blunted insulin response, thereby resulting in greater fat utilisation when compared with the faster-digesting WP and CHO. Therefore, it is plausible that in addition to improving REE, CP may be an ideal macronutrient for utilising fat as a fuel source in the late evening.
A few limitations exist and need to be addressed. Differences in taste, serving size and texture of the PLA may have confounded the subjective feelings of hunger, satiety and desire to eat. However, the decision to add a non-energy-containing PLA was necessary for the measurements of VO2 and REE to identify differences between energy intake and not consuming energy before sleep. Likewise, although we randomised the order of each experimental trial and required a 48–72 h washout period between laboratory visits, it is possible that the order of treatments may have confounded our findings. Furthermore, although purely speculation, the physical differences between the PLA and WP/CP/CHO supplements would probably have little impact on metabolic measurements via indirect calorimetry. In addition, the question remains as to whether the energy load of the ingested supplements was offset by the increase in REE over the sleeping hours or the next day. Therefore, future research should investigate metabolism through the overnight period and for a duration longer than 60 min the following morning to fully understand any weight management implications of the present findings. As very limited research exists on whether it is detrimental to consume energy-containing products before sleep, the present findings begin to identify what active people should consume in the late evening before sleep.
Conclusions
The majority of studies have compared the effects of different proteins, macronutrients and energy loads when consumed in the morning. The present study is the first to investigate the effects of WP, CP, CHO and PLA when consumed before sleep on morning satiety and resting metabolism.
We conclude that consumption of 586 to 628 kJ (140–150 kcal) of WP, CP and CHO before sleep increases morning REE in healthy, physically active young men, while that of a PLA does not. Our findings contradict the popular belief that it is advantageous to limit energy intake in the evening. In fact, protein consumption before sleep after an evening resistance exercise bout has been shown to increase muscle protein synthesis overnight in young healthy men( Reference Res, Groen and Pennings 17 ), and the results of the present study suggest that regardless of the macronutrient type, energy intake of 586–628 kJ (140–150 kcal) before sleep is more beneficial than not eating. Although the aforementioned study( Reference Res, Groen and Pennings 17 ) provided a stimulus for muscle protein synthesis (resistance exercise), the present results and those of Res et al. ( Reference Res, Groen and Pennings 17 ) suggest a plausible synergistic benefit of resistance exercise and late-evening protein consumption to increase both overnight muscle protein synthesis and REE. Therefore, night-time protein and CHO consumption may be an effective nutritional strategy to further enhance recovery and improve resting metabolism with a minimal effect on the feelings of satiety. In addition, it is convenient to hypothesise that the improvement in morning resting metabolism may further aid in the maintenance of and/or improvement in body composition and thereby provide a competitive advantage in healthy, physically active young men. Future studies should investigate the impact of liquid energy intake v. solid energy intake and combinations of the macronutrients for the most optimal metabolic milieu. In addition, long-term studies of night-time feeding are warranted.
Acknowledgements
The authors thank the participants for their dedication and participation in the present study. The study was funded by the Florida State University. M. J. O. conceived and designed the study, secured funding for the project, and provided oversight for data collection, analysis and manuscript preparation. T. A. M. carried out participant recruitment and data collection and assisted with manuscript preparation. S. K. F. assisted with data collection and recruitment. A. W. K. helped with manuscript preparation. L. B. P. helped with the study design and manuscript preparation. All authors read and approved the final manuscript. None of the authors has financial or other interests concerning the outcomes of the investigation. The authors declare that they have no competing interests.






