Essential fatty acids, including n-6 and n-3 PUFA, cannot convert into each other in the body. Hence, PUFA are crucial components of the food or diet( Reference Lim, Calon and Morihara 1 , Reference Simopoulos 2 ). It has been widely accepted that the present Western diet is low in n-3 fatty acids with a ratio of n-6:n-3 ranging from 15:1 to 20:1, instead of 1:1, while a value as much as possibly close to 1:1 is considered protective against degenerative pathologies( Reference Simopoulos 3 , Reference Kobayashi, Barnard and Henning 4 ). Both n-6 and n-3 PUFA can regulate gene expression: n-3 PUFA exert suppressive effects on chronic diseases; conversely, n-6 PUFA increase the concentrations of inflammatory mediators( Reference Simopoulos 2 , Reference Hibbeln, Nieminen and Blasbalg 5 ). On the one hand, it has been hypothesised that diets with high ratios of n-6:n-3 PUFA may increase the production of inflammatory mediators and lead to the pathology of the metabolic syndrome, such as cognitive impairment, Alzheimer's disease and type 2 diabetes( Reference Gamoh, Hashimoto and Hossain 6 – Reference Weaver, Ivester and Seeds 10 ). On the other hand, diets with higher ratios of n-6:n-3 fatty acids may lead to the pathology of the metabolic syndrome( Reference Hibbeln, Nieminen and Blasbalg 5 ). Therefore, lowering the n-6:n-3 PUFA ratio in diets is beneficial for the health of animals and humans.
A lower n-6:n-3 PUFA ratio is required for the prevention and management of chronic diseases( Reference Simopoulos 2 ). Some previous studies have suggested that an n-6:n-3 PUFA ratio of 5:1 suppresses inflammation in patients with asthma( Reference Simopoulos 3 ). It should be noted that the biological effects of n-6 and n-3 PUFA are not always in opposition. It is widely accepted that the n-6-derived lipoxins also exert anti-inflammatory effects( Reference Simopoulos 3 ). Due to their opposing and coordinative effects, a proper balance between n-6 and n-3 fatty acids in the diet is very important to maintain the optimum growth and development of animals and also the health of humans( Reference Dutta-Roy 11 ).
The morphology and physiology of the organs of humans and pigs are similar. Thus, the pig is an excellent animal model for studying human nutrition and metabolism. In the present study, we used a pig model to investigate the optimal dietary ratios of n-6:n-3 PUFA that regulate lipid metabolism and inflammation.
Materials and methods
Animals and diets
All procedures followed in the present experiment were approved by the committee on animal care of the Institute of Subtropical Agriculture, the Chinese Academy of Sciences.
A total of ninety-six male cross-bred (Large White × Landrace) pigs with a similar initial weight (73·8 (sem 1·6) kg) were chosen and divided into four groups using a randomised complete block design based on body weight, with six replicates (pens) per group and four pigs per replicate. The pigs in the four groups were fed isoenergetic diets (3 % fat) with different n-6:n-3 ratios, prepared using 3·00, 1·50, 0·75 and 0·30 % of linseed oil to replace equivalent amounts of soyabean oil to make the dietary n-6:n-3 ratios of the four diets about 1:1, 2·5:1, 5:1 and 10:1, respectively. The composition and nutrient levels of the four diets are listed in Table 1. All the pigs had ad libitum access to diets and water and consumed the diets for 2 months.
Table 1 Composition and nutrient levels of the diets (air-dry basis, %)
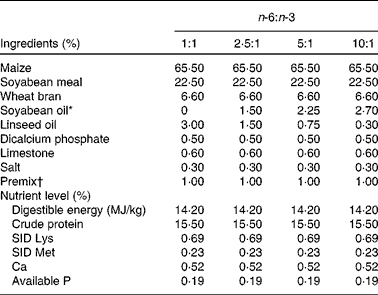
SID, standardised ileal digestible.
* To replace equivalent amounts of soyabean oil, 3·00, 1·50, 0·75 and 0·30 % of linseed oil were used, making the dietary n-6:n-3 ratios about 1:1, 2·5:1, 5:1 and 10:1, respectively (see Table 2).
† Premix provided per kg diet: retinol acetate, 13 500 IU; cholecalciferol, 3600 IU; dl-α-tocopherol acetate, 15 IU; thiamin, 3·0 mg; riboflavin, 7·8 mg; cobalamin, 0·024 mg; pyridoxine, 3·0 mg; menadione, 3·0 mg; pantothenic acid, 150 mg; niacin, 30 mg; choline, 600 mg; folic acid, 1·5 mg; biotin, 0·045 mg; Cu (as CuSO4.5H2O), 10 mg; Fe (as FeSO4.7H2O), 80 mg; Zn (as ZnSO4.7H2O), 80 mg; Mn (as MnSO4.H2O), 10 mg; Se (as Na2SeO3), 0·30 mg; I (as KI), 0·30 mg.
Sample collection
Body weights and feed intake of the pigs were recorded after an overnight fast to calculate weight gain and feed conversion.
From each replicate, one pig was chosen and killed at the end of the feeding test. Blood samples were collected via jugular vein puncture into 10 ml tubes, and serum was separated by centrifugation at 2000 g for 15 min at 4°C and then stored at − 20°C until analysis. The pigs were electrically stunned, exsanguinated and eviscerated. Immediately, samples (about 5 g) of the longissimus lumborum muscle and subcutaneous adipose tissue dissected from the left side of the carcasses were placed in liquid N2 and then stored at − 80°C until further analyses. Later, skeletal muscle and fat were dissected from the right side of the carcasses and weighed separately. The weights were used to calculate the total percentages of these components in the carcasses.
Measurement of the concentrations of secreted adipokines by ELISA
The serum concentrations of IL-6 (R&D), TNF-α (Endogen), IL-1β, leptin, total adiponectin (Uscn) and insulin (Mercodia) were quantified using ELISA kits for porcine assay according to the manufacturers' instructions. All the samples were measured in six replicates.
Real-time PCR
Total RNA was extracted from the harvested tissue using the TRIzol reagent (Invitrogen). Primers for the selected genes (Table 2) were designed using the Oligo 6.0 software. RT was performed using the AMV Reverse Transcriptase Kit (Promega). The relative expression levels of the target genes were determined using quantitative real-time PCR, performed with an ABI 7900 PCR system (ABI Biotechnology). The final volume of the reaction mixtures (20 μl) contained diluted complementary DNA and SYBR Green I (Molecular Probes) as a PCR core reagent. β-Actin was used as a housekeeping gene or an internal control to normalise the expression of target genes.
Table 2 Primers used for real-time PCR

T A, annealing temperature; PI3Kα, phosphoinositide-3-kinase-α; FATP-1, fatty acid transport protein-1.
The relative quantification of gene amplification by RT-PCR was performed using the value of the threshold cycle (C
t). The comparative C
t value method using the formula
![]() $$2^{ - \Delta \Delta C _{t}} $$
was employed to quantify the expression levels of phosphoinositide-3-kinase-α (PI3Kα), fatty acid transport protein-1 (FATP-1), PPARγ, IL-1β, TNF-α and IL-6 relative to those of β-actin using the following formula:
$$2^{ - \Delta \Delta C _{t}} $$
was employed to quantify the expression levels of phosphoinositide-3-kinase-α (PI3Kα), fatty acid transport protein-1 (FATP-1), PPARγ, IL-1β, TNF-α and IL-6 relative to those of β-actin using the following formula:
Western blotting
Tissue samples (about 500–800 mg) were powdered in liquid N2 to extract total protein. Approximately 30 μg of the protein sample were size-fractionated on SDS–PAGE gel and transferred onto polyvinylidene difluoride membranes (Millipore) under the conditions of 30 mA and 4°C overnight. Later, the membranes were blocked with 5 % bovine serum albumin (BSA) for 1 h and then probed overnight at 4°C with the antibodies against FATP-1 (ab69458; Abcam) at 1:800 dilution and PPARγ (#2435; Cell Signaling Technology) and PI3Kα (#4255; Cell Signaling Technology) at 1:1000 dilution. The membranes were then rinsed with Tris-buffered saline plus 0·1 % Tween 20 three times and incubated with peroxidase-conjugated goat anti-rabbit or anti-mouse IgG for 1 h at 1:5000 dilution at room temperature; β-actin monoclonal antibody (sc47778) at 1:2000 dilution was used to normalise the amount of proteins (Santa Cruz Biotechnology). The protein bands were visualised using a chemiluminescent reagent. The density of the protein bands was determined using the Alpha Imager 2200 software (Alpha Innotech Corporation).
Statistical analyses
All the results are expressed as means with their standard errors. Statistical analyses were carried out using one-way ANOVA, SAS 8.2 (SAS Institute, Inc.), followed by a Tukey test of multiple comparisons. In case of a P value < 0·05, differences were considered to be statistically significant.
Results
Effects of dietary n-6:n-3 PUFA ratios on the growth performance and body composition of pigs
The fatty acid contents of the experimental diets are listed in Table 3. The measured values coincided better with the calculated values. The growth performance and body composition of pigs fed diets with different n-6:n-3 PUFA ratios are summarised in Table 4. Compared with those of the other groups, the body weight and daily weight gain of pigs fed the diet with an n-6:n-3 PUFA ratio of 5:1 were increased significantly (P< 0·05), while the daily intake and feed conversion rate of this group were decreased (P< 0·05). However, the group fed the diet with an n-6:n-3 PUFA ratio of 1:1 had high muscle mass and low adipose tissue mass. We speculated that an optimal n-6:n-3 PUFA ratio could regulate the crosstalk between the muscle and adipose tissue of pigs.
Table 3 Fatty acid composition of the diets
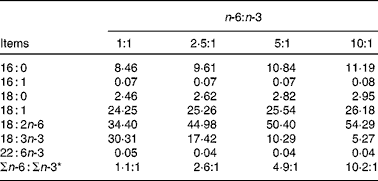
* n-6:n-3 = (18 : 2)/(18 : 3+22 : 6).
Table 4 Effect of dietary n-6:n-3 PUFA ratios on the growth performance of pigs
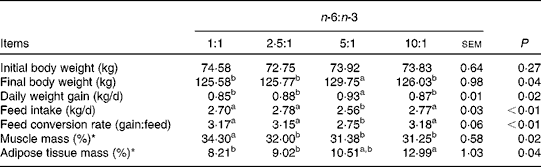
a,bValues with unlike letters within a row were significantly different (P< 0·05).
* The ratio represents the muscle or adipose tissue mass:carcass weight.
Effects of different n-6:n-3 PUFA ratios on serum glucose and cytokine concentrations
As shown in Table 5, the concentrations of glucose, TNF-α and insulin were not different among the treatment groups. The concentrations of IL-6 and IL-1β of pigs fed the diet with an n-6:n-3 PUFA ratio of 1:1 were decreased by 12·3 and 37·9 % (P <0·05), respectively, compared with those fed diets with an n-6:n-3 PUFA ratio of 10:1. Furthermore, the serum concentrations of adiponectin of pigs fed the diet with an n-6:n-3 PUFA ratio of 1:1 were also decreased by 13·5 % compared with those of pigs fed the diet with an n-6:n-3 PUFA ratio of 10:1 (P <0·05); on the contrary, the concentration of leptin of this group was increased by 16·4 % (P <0·05).
Table 5 Effect of dietary n-6:n-3 ratios on serum glucose and cytokine concentrations of pigs
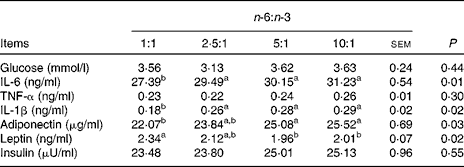
a,bValues with unlike letters within a row were significantly different (P< 0·05).
Effects of dietary n-6:n-3 PUFA ratios on the gene expression levels of pigs
The expression levels of genes in the muscle and adipose tissue of pigs are shown in Fig. 1(A) and (B). The expression levels of PI3Kα mRNA were lower (P <0·05) in the groups fed diets with n-6:n-3 PUFA ratios of 1:1 and 2·5:1, and there was no difference between these two groups (P>0·05). The expression levels of the FATP-1 gene in the muscle and adipose tissue of pigs fed diets with an n-6:n-3 PUFA ratio of 1:1 were the lowest (P <0·05), and those fed diets with n-6:n-3 PUFA ratios of 1:1 and 2·5:1 exhibited down-regulated expression levels of the PPARγ gene in the muscle and adipose tissue (P <0·05); also, there was no difference (P <0·05) between these two groups. Interestingly, the diet with an n-6:n-3 PUFA ratio of 1:1 markedly down-regulated the expression levels of IL-1β, TNF-α and IL-6 genes in the skeletal muscle and adipose tissue of pigs (P <0·05).

Fig. 1 Relative expression levels of phosphoinositide-3-kinase-α (PI3Kα), fatty acid transport protein-1 (FATP-1), PPARγ, IL-1β, TNF-α and IL-6 mRNA in the (A) muscle and (B) adipose tissue of pigs fed diets with n-6:n-3 PUFA ratios of 1:1 (□), 2·5:1 (![]() ), 5:1 (
), 5:1 (![]() ) and 10:1 (■). Real-time PCR method was employed. Values are means (n 6), with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05).
) and 10:1 (■). Real-time PCR method was employed. Values are means (n 6), with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05).
Effect of dietary n-6:n-3 PUFA ratios on the protein expression levels of pigs
The relative expression levels of PI3Kα, FATP-1 and PPARγ proteins are shown in Fig. 2(A) and (B). The expression level of the PI3Kα protein was higher in the muscle of pigs fed the diet with an n-6:n-3 PUFA ratio of 10:1 (P <0·05). The trend of the expression levels of the FATP-1 protein was the same as those of the gene in the muscle. However, the trend of the expression levels of the FATP-1 protein in the adipose tissue was the reverse. The diets with n-6:n-3 PUFA ratios of 1:1 and 2·5:1 significantly down-regulated the expression levels of the PPARγ protein in the muscle and adipose tissue (P <0·05), also partially corresponding to the expression levels of the gene.

Fig. 2 Relative expression levels of phosphoinositide-3-kinase-α (PI3Kα), fatty acid transport protein-1 (FATP-1) and PPARγ proteins in the (A) muscle and (B) adipose tissue of pigs fed diets with different n-6:n-3 PUFA ratios. Western blotting method was employed. Lanes 1, 2, 3 and 4 represent n-6:n-3 PUFA ratios of 1:1 (□), 2·5:1 (![]() ), 5:1 (
), 5:1 (![]() ) and 10:1 (■), respectively. Values are means (n 6), with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05).
) and 10:1 (■), respectively. Values are means (n 6), with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05).
Discussion
In the present study, with an accompanying decline in the average daily feed intake and feed conversion rate, the final body weight and daily gain of pigs fed the diet with an n-6:n-3 PUFA ratio of 5:1 improved significantly. This result is in agreement with earlier reports showing that a diet with a lower n-6:n-3 PUFA ratio, rich in n-3 PUFA, is beneficial for the growth performance and health of animals( Reference Newman, Bryden and Fleck 12 – Reference Drevon 15 ).
The main components of adipose tissue are fatty acids, which may influence the expression of adipokines, such as adiponectin and leptin( Reference Drevon 15 ). Interestingly, the results of the present experiment showed that the serum concentrations of adiponectin of pigs decreased gradually as the dietary n-6:n-3 PUFA ratio decreased. Adiponectin is an adipokine exclusively derived from the adipose tissue( Reference Stefan, Wahl and Fritsche 16 , Reference Neschen, Morino and Rossbacher 17 ). It has been reported that fish oil rich in n-3 PUFA increases the serum concentrations of adiponectin in mice 2–3-fold in a dose-dependent manner and also in a PPARγ-dependent manner( Reference Neschen, Morino and Rossbacher 17 ). However, the present results showed that a low n-6:n-3 PUFA ratio could reduce the serum concentrations of adiponectin. The results led us to hypothesise that the serum concentrations of adiponectin are affected by different ratios of dietary n-6:n-3 PUFA. Leptin circulates in the body at a concentration highly correlated with white adipose tissue mass and may be of great importance in the regulatory action on body fat( Reference Drevon 15 , Reference Lago, Dieguez and Gómez-Reino 18 ). It has been shown that the serum concentrations of leptin are significantly reduced in mice fed diets with an n-6:n-3 PUFA ratio of 1:1, but these are not significantly reduced in mice fed diets with n-6:n-3 PUFA ratios of 5:1, 10:1 and 20:1( Reference Xu, Fan and Zhu 19 ). In the present study, the concentrations of leptin of the group fed the diets with n-6:n-3 PUFA ratios of 1:1 and 2·5:1 were higher, indicating that the optimal ratio may vary in different animal models. However, no difference in the serum concentrations of insulin was observed in the present study. We speculated that n-6:n-3 PUFA ratios could stimulate the negative feedback regulatory mechanism of adiponectin and leptin.
Immune stimulation in the rearing environment results in the production of potent pro-inflammatory cytokines, which antagonise anabolic growth factors and thus suppress growth. IL-6, IL-1β and TNF-α, which are all inflammatory cytokines, initiate the production of an array of inflammatory mediators, thus leading to an inflammatory response. The concentrations of these cytokines are increased on increasing n-6 fatty acid intake and decreased on increasing n-3 fatty acid intake in bovine chondrocytes and in mouse kidney, spleen and peritoneal macrophages, as well as in human monocytes( Reference Tai and Ding 20 ). The circulating levels of IL-6 might reflect, at least in part, the production of IL-6 in the adipose tissue, although it is also secreted by the exercising muscle( Reference Lafontan and Langin 21 ). The concentrations of IL-6 decrease by 10·5 % on altering the n-6:n-3 ratio to 1·3( Reference Rallidis, Paschos and Liakos 22 ). Moreover, the concentrations of TNF-α decline significantly by 30 % in response to a flaxseed oil diet rich in n-3 PUFA and decrease by 74 % after fish oil supplementation( Reference Caughey, Mantzioris and Gibson 23 ). The present results also indicated that higher n-3 PUFA could reduce the serum concentrations of IL-6 as well as IL-1β, but not of TNF-α. Additionally, we also found that the expression levels of IL-6, IL-1β and TNF-α mRNA in the skeletal muscle and adipose tissue of pigs fed the diet with an n-6:n-3 PUFA ratio 1:1 were markedly down-regulated. Numerous studies have reported that n-3 PUFA can decrease the production of these inflammatory cytokines( Reference Weaver, Ivester and Seeds 10 , Reference Calder 24 , Reference Calder 25 ). It has been shown that an optimal n-6:n-3 PUFA ratio could regulate several cytokines to reduce inflammatory events in the body.
The PI3K pathway controls essential cellular functions such as signal transduction, cytoskeletal dynamics and membrane trafficking( Reference Lindmo and Stenmark 26 ). The expression levels of the PI3Kα gene in mononuclear cells of healthy human subjects have been reported to decrease after supplementation with fish oil( Reference Weaver, Ivester and Seeds 10 ). In the present study, the expression levels of PI3Kα gene and protein in the muscle and adipose tissue of pigs fed the diet with an n-6:n-3 PUFA ratio of 1:1 were the lowest and the PI3K pathway was activated. In mammals, FATP-1 transports long-chain fatty acids actively across adipocyte cell membranes. In the present study, it was found that the expression levels of FATP-1 mRNA and protein in the muscle and adipose tissue were down-regulated significantly in pigs fed the diet with a lower n-6:n-3 PUFA ratio. We speculated that n-3 PUFA could suppress adipogenic processes by down-regulating the expression levels of FATP-1 and the optimal dietary ratios of n-6:n-3 PUFA might be 1:1 and 5:1. PPARγ regulates genes involved in adipocyte differentiation and lipogenesis, while n-3 PUFA and their metabolites have been shown to suppress the transcription of lipogenic genes by functioning as natural ligands for PPAR( Reference Madsen, Petersen and Kristiansen 27 – Reference Edwards and O'Flaherty 31 ). Interestingly, n-3 PUFA and their metabolites can activate the extracellular signal-regulated kinase pathway( Reference Feige, Gelman and Michalik 29 , Reference Edwards and O'Flaherty 31 , Reference Camp, Tafuri and Leff 32 ), which primarily regulates cellular growth and differentiation( Reference Dorman and Johnson 33 , Reference Lee, Jeong and Oh 34 ). Some previous studies have already demonstrated that PPARγ ligands inhibit the production of IL-6, TNF-α and IL-1β( Reference Moraes, Piqueras and Bishop-Bailey 28 , Reference Jiang, Ting and Seed 35 , Reference Escher and Wahli 36 ) and that TNF-α can inhibit adipocyte differentiation and adipogenesis by suppressing the expression of the PPARγ gene( Reference Tai and Ding 20 , Reference Escher and Wahli 36 ). In the present study, the expression levels of PPARγ gene and protein in both the muscle and adipose tissue of pigs fed the diets with n-6:n-3 PUFA ratios of 1:1 and 2·5:1 were markedly reduced. It was observed that a diet with a lower n-6:n-3 PUFA ratio could reduce the expression levels of PPARγ, which further suppress the transcription of lipogenic genes and lipogenesis.
Conclusion
On the whole, n-6:n-3 PUFA ratios regulate lipid metabolism and inflammation differently and the optimal ratios are 1:1 to 5:1, which vary based on the roles under considerations. Optimal n-6:n-3 PUFA ratios could inhibit immune stimulation to ensure the availability of more energy and nutrients for high performance and homeostatic pathways. We speculated that there was a common pathway shared by energy metabolism and inflammation modulation. However, further research is necessary to confirm the results and to illustrate the underlying metabolic pathways.
Acknowledgements
The present study was jointly supported by the National Basic Research Program of China (2013CB127305, 2012CB124704), the Nanjing Branch Academy of Chinese Academy of Science and Jiangxi Province Cooperation Project, the National Nature Science Foundation of China (31001015, 31110103909) and the Project of Institute of Subtropical Agriculture, the Chinese Academy of Sciences (ISACX-LYQY-QN-1104). None of the funders had any role in the design and analysis of the study and in the writing of this article.
The authors' contributions are as follows: Y. Y., F. L. and L. L. were in charge of the whole trial; Y. D. and F. L. wrote the manuscript; J. F. and X. S. assisted with the animal trial and biochemical analyses.
The authors have no conflicts of interest to declare.









