The edible dry bean, Phaseolus vulgaris L., is a pulse crop, i.e. a grain legume that serves as a staple food crop for populations around the world(1). Since ancient times, pulses have been combined with cereal crops, primarily wheat, rice and corn, or tubers such as potato or cassava to provide a balanced source of protein and carbohydrate and a nutritionally adequate diet when combined with seed oils, fruit and vegetables(Reference Geil and Anderson2, Reference Gepts3). In this setting, the established ratio of cereal grains to pulses to meet nutritional requirements is 2:1; however, over the last 50 years both production and consumption patterns, globally, have changed to 8:1(1, Reference Leterme4). In developing countries, the consumption of pulses declines with a rise in socio-economic status since pulses crops, particularly dry beans, are considered ‘poor man's food’(Reference Leterme4). In Westernised societies such as the USA, animal sources of protein have largely displaced pulse crops from the diet and even when individuals choose not to eat animal products, they frequently use soya-derived protein as the legume source(Reference Mitchell, Lawrence and Hartman5). While soya has many desirable properties, it is an oil-seed legume with markedly different profiles of macro- and micronutrients and bioactive food components compared with dry beans. While the pandemic of obesity that has swept the globe over the last 50 years is now receiving considerable attention(6, 7), as are the associated increases in the incidence of and mortality due to type 2 diabetes, heart disease and cancer, there has been virtually no scrutiny of the potential association between the decline in edible dry bean consumption and the global rise in obesity and its associated metabolic disorders(Reference Thompson, Thompson and Sparks8). The work reported in the present paper represents a first step in addressing this issue at the molecular level.
A small but growing body of literature that was recently reviewed(Reference Geil and Anderson2, Reference Bennink and Rondini9) indicates that increased consumption of dry beans is protective against the occurrence of type 2 diabetes, CVD and certain types of cancer. In addition, our laboratory and others have reviewed evidence that outlines the metabolic effects that are common among these diseases(Reference Thompson, Thompson and Sparks8, Reference Eyre, Kahn and Robertson10). All three diseases are associated with alterations in glucose homeostasis, chronic inflammation and cellular oxidation(Reference Frasca, Pandini and Sciacca11, Reference Hartman, Albert and Zhang12). What have been less emphasised are the alterations in lipid metabolism that also accompany these chronic diseases(Reference Zhang, Lanza and Kris-Etherton13). Relative to mechanisms of effect, there are a paucity of specific hypotheses about how dry beans exert protective effects. Recently, our laboratory reported that dry bean consumption inhibited the occurrence of experimentally induced breast cancer and provided evidence that dry bean intake affected both host systemic and cell autonomous mechanisms(Reference Thompson, Brick and McGinley14, Reference Thompson, Thompson and Brick15). Evidence was also reported that showed dry bean varieties differed in anticancer activity in a manner that could be predicted by their genetic heritage(Reference Thompson, Brick and McGinley14). Moreover, several lines of evidence point to potential effects of dry beans on lipid metabolism in accounting for health benefits(Reference Bennink and Rondini9, Reference Duane16–Reference Guillon and Champ18). Consequently, the objective of the experiments reported in the present paper was to determine if the pattern of cellular metabolites detected in breast tissue harvested from tumour-free animals in our previously reported carcinogenesis experiments or in the mammary carcinomas that were induced in those experiments was consistent with the hypothesis that dry bean consumption alters lipid metabolism. Three questions were addressed: (1) how does dry bean consumption affect metabolite patterns in mammary gland from cancer-free animals and in the mammary carcinomas that occurred in tumour-bearing animals; (2) what pathways of cell regulation are inferred by the metabolite patterns; and (3) do the patterns of metabolites differ by dry bean genotype?
Methods
Materials and chemicals
The following chemicals and materials were used as received from Thermo Fisher: chloroform, methanol, acetonitrile and HPLC-grade water.
Source of tissue for analysis
The mammary gland and mammary carcinomas evaluated in the present study were obtained from a previously reported experiment(Reference Thompson, Brick and McGinley14). Briefly, female Sprague–Dawley rats were obtained from Taconic Farms at age 20 d. Animal rooms were maintained at 22 ± 2°C with 50 % relative humidity and a 12 h light–12 h dark cycle. At age 21 d, rats were injected with 1-methyl-1-nitrosourea (50 mg/kg body weight, intraperitoneally), as previously described. For week 1 of the study, rats were housed three per cage in solid-bottomed polycarbonate cages equipped with a food cup; they were given free access to American Institute of Nutrition (AIN)-93G control diet. At 7 d following carcinogen injection, all rats were randomised to diet groups based on body weight: control diet, small red (SR) bean, navy (NV) bean, or white kidney (WK) bean diet. Beans were incorporated at 60 % (w/w). Rats were fed their assigned diets ad libitum until the end of the study at 46 d post-carcinogen injection. The post-initiation design of this experiment simulates the promotion and progression events of the disease process, which is highly relevant to women at increased risk for breast cancer and to breast cancer survivors. The tissue was harvested from animals following an overnight fast of 12–14 h. The work followed guidelines approved by the Colorado State University Animal Care and Use Committee.
Because of inter-individual variability in the metabolite profiles among animals, ten mammary glands from ten different rats per treatment group (WK, NV, SR, control) were used for analysis (200 mg tissue per sample). The mammary glands were taken from animals that had no palpable tumours. Similarly, ten carcinomas per treatment condition (200 mg tissue per sample) were also evaluated. Cancer incidence and multiplicity per treatment group were 95, 71, 66 and 63 % and 3·23, 1·55, 1·67 and 1·14 carcinomas per rat for control, SR, NV and WK bean diet groups, respectively(Reference Thompson, Brick and McGinley14). The 1-methyl-1-nitrosourea-induced model for breast cancer is one of the most widely used laboratory systems for studying this disease process in a pre-clinical setting. Histologically, the carcinomas induced and their pre-malignant precursors have many similarities to the human disease. A histological comparison of the lesions induced in this model relative to those observed in the human disease has been published by our laboratory(Reference Thompson, Singh and McGinley19).
Sample preparation
Before extraction, the mammary gland and tumour tissue samples were ground under liquid N2 and 100 mg of each sample placed in separate 1·5 ml microcentrifuge tubes. Metabolite extraction was carried out using a modified Bligh and Dyer method published by Sana et al. (Reference Sana, Waddell and Fischer20). To account for differential solubility of various compounds and to extract the largest number of compounds possible from the mammary and tumour tissue samples, extractions were carried out at pH 2 and 9(Reference Hendriks, Uges and Franke21). Following extraction, samples were dried completely using a Speedvac under ambient temperature and stored at − 80°C until analysis. Before liquid chromatography (LC)–MS analysis, samples were redissolved in 100 μl 50:50 methanol–water (pH 7, milli-Q) by adding 50 μl methanol followed by 50 μl water with a brief vortexing step after each addition. A quantity of 900 μl of a 75:25 mixture of acetonitrile–water was added at room temperature followed again by a brief vortexing step. Samples were placed in a − 20°C freezer for 1 h, centrifuged and the supernatant fraction carefully removed from any pelleted material that was present, transferred to new 1·8 ml tubes, and dried using a Speedvac. The samples were again redissolved in 50:50 methanol–water by adding 50 μl methanol followed by 50 μl Milli-Q water (containing 0·4 % (v/v) acetic acid) and vortexed after each solvent was added. The 100 μl was transferred to a fresh 1·8 ml centrifuge tube containing 0·2 μm ultrafiltration membrane. Extracts were centrifuged at 4000 rpm, 4°C for 20 min. The liquid below the membrane was transferred to a tube containing a 10 kDa filter, centrifuged at 4000 rpm, 4°C for 20 min or until the majority of liquid had passed through the membrane. The acidic (pH 2) and basic (pH 9) samples were combined in equal volumes in LC vials for analysis.
Sample analysis using liquid chromatography–MS
Extracts were separated with a Zorbax SB-Aq column 2·1 × 150 mm on an Agilent 1200 LC. Injections (5 μl) were made from 100 μl sample volumes. A flow rate of 0·6 ml/min was used with a 2 to 98 % linear gradient of water–methanol over 13 min followed by a 7 min solvent hold. A 0·2 % solution of acetic acid was used as a mobile phase modifier. An Agilent 6510 Quadrupole Time-of-Flight LC–MS with an electrospray ion source was used to acquire accurate-mass profiling MS data for tissue samples. Data were collected in both positive and negative ionisation modes over the range of 50 to 1500 m/z.
Multivariate analysis
Initial data processing was carried out using the Molecular Feature Extraction algorithm in MassHunter Workstation software (Agilent Technologies). The retention time/mass pairs generated by MassHunter Workstation were exported as CEF files for subsequent analysis in Agilent Mass Profiler Professional (MPP) software. MPP was used for retention time and mass abundance adjustments. The data files were aligned to correct for retention time drift and raw intensities were used in statistical analysis. Any intensity less than 1 was set to 1 before analysis. Samples were imported into MPP using the guided workflow option. The following filter parameters were used for importing sample data: minimum absolute abundance was set at 1000, minimum number of ions was set to 2, retention time greater than 0·3 min, and a mass between 50 and 1500 m/z. Furthermore, only those features present in eight out of ten replicates, in one or more groups were analysed. The features were then analysed to determine differential features across groups using one-way ANOVA (P < 0·05, Benjamini–Hochberg false discovery rate (FDR) multiple testing correction) and Kruskal–Wallis one-way ANOVA(Reference Benjamini and Hochberg22, Reference Sokal and Rohlf23). The resulting mass list was exported as a CEF file for recursive analysis. Datasets were re-mined using the Find by Formula algorithm in MassHunter with a mass window of 10 parts per million (ppm) and a retention time window of 0·2 min. The general integrator was applied for recursive analysis. The resulting data files were re-imported into MPP for analysis using parameters identical to the original import described above. Unsupervised principal component (PC) analysis (PCA) with mean centring and scaling was carried out to visualise differences in metabolite profiles of the four treatment groups. Differential abundances of metabolites between WK bean-fed, NV bean-fed or SR bean-fed v. control-fed animals were determined using an unpaired t test with Benjamini Hochberg FDR multiple test correction (P < 0·05)(Reference Hochberg and Tamhane24). Inclusion lists were generated for those features found to be at least 2-fold higher in all three bean-fed groups or those features found to be at least 2-fold higher in the control compared with all three bean-fed groups. Feature lists were queried against the Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg) using MassTRIX (http://masstrix.org) with a mass error of 7 ppm and Rattus norvegicus as the model organism in order to assign tentative compound identities and active pathways.
Results
The effect of bean diets on the mammary gland metabolite profile
Using an unbiased analytical approach, i.e. not targeted to a specific class of chemicals, tissue samples were analysed in both positive and negative ionisation mode over the m/z range of 50–1500. Within this m/z range, 495 and 1775 features were detected in positive and negative ionisation modes, respectively. Applying a one-way ANOVA before PCA reduced these numbers to 201 and 253 features (P < 0·05). As shown in Fig. 1(A), the control group is clearly separated from the bean diet groups in ESI-positive mode, with 95·42 % of the total variance being explained by the first three components (PC1, 77·11 %; PC2, 12·28 %; PC3, 6·03 %). Despite the large amount of variation explained by the first three components, the three bean-fed groups are not clearly distinguishable based on the features detected in ESI-positive mode. It is also the case that the metabolites in ESI-negative mode clearly separate the control-fed animals from the bean-fed groups (Fig. 1(B)), where 55·73 % of the total variance is explained by the first component, with PC2 and PC3 explaining an additional 24·55 and 12·84 % of the total variance, respectively. In contrast to the metabolite profiles generated in ESI-positive mode, the WK bean-fed group is clearly distinguishable from both the control and the other two bean-fed groups. Of the 201 features detected in positive ionisation mode, 186 were overlapping in all three bean groups using control as a baseline, with only twenty-two being tentatively identified after submission to MassTRIX using R. norvegicus (brown rat) as the model organism (Table 1). In negative ionisation mode, 192 features were found at consistently higher levels in NV, SR and WK bean-fed animals when compared with the control group and nineteen ions were identified by mapping the metabolites to pathways in KEGG using MassTRIX (Table 1). Small molecules elevated in the control group were also explored. In this case, a total of thirty-nine molecules were elevated in the control-fed group relative to all three bean-fed groups (twelve and twenty-seven for positive and negative mode, respectively). Of these molecules, nine were tentatively identified using MassTRIX (Table 1).

Fig. 1 Principal component analysis (PCA) scores plot for the comparison of mammary gland tissue from control and bean-fed rats based on (A) positive ionisation mode and (B) negative ionisation mode. PCA shows a separate clustering based on control (![]() ), white kidney (
), white kidney (![]() ), small red (
), small red (![]() ) and navy (
) and navy (![]() ) bean diets.
) bean diets.
Table 1 List of identified metabolites in mammary gland*

RT, retention time; ESI − , electrospray ionisation negative mode; ESI+, electrospray ionisation positive mode.
* List based on MassTRIX query of Kyoto Encyclopedia of Genes and Genomes (KEGG).
† Indicates feature is increased in bean-fed animals ( ↑ ) or decreased in bean-fed animals ( ↓ ) relative to the control group.
‡ Metabolite type assigned based on KEGG search using MassTRIX. Total number of masses = 454, metabolite types identified = 41/454 or 9·0 %, non-identified metabolite types = 413/454 or 91·0 % (not shown).
Bean diets down-regulate TAG synthesis in the mammary gland
A total of five TAG precursors were tentatively identified using the KEGG database in all diet groups (Fig. 2). Of the five TAG precursors, three were significantly reduced in the mammary gland tissue of bean-fed animals when compared with the control group (Kruskal–Wallis; P < 0·05). The molecule putatively identified as palmitic acid was the most abundant TAG precursor in all of the diet groups evaluated. 2-Tetradecenoic acid was also significantly reduced in all bean-fed animals while 3,7-octadecadiynoic acid was the only elevated TAG putatively identified in the three bean-fed groups. Stearic acid was found to be significantly reduced in only the NV bean-fed and WK bean-fed animals. The decrease in 11-hydroxypalmitic acid was not statistically significant in any of the three bean-fed groups.
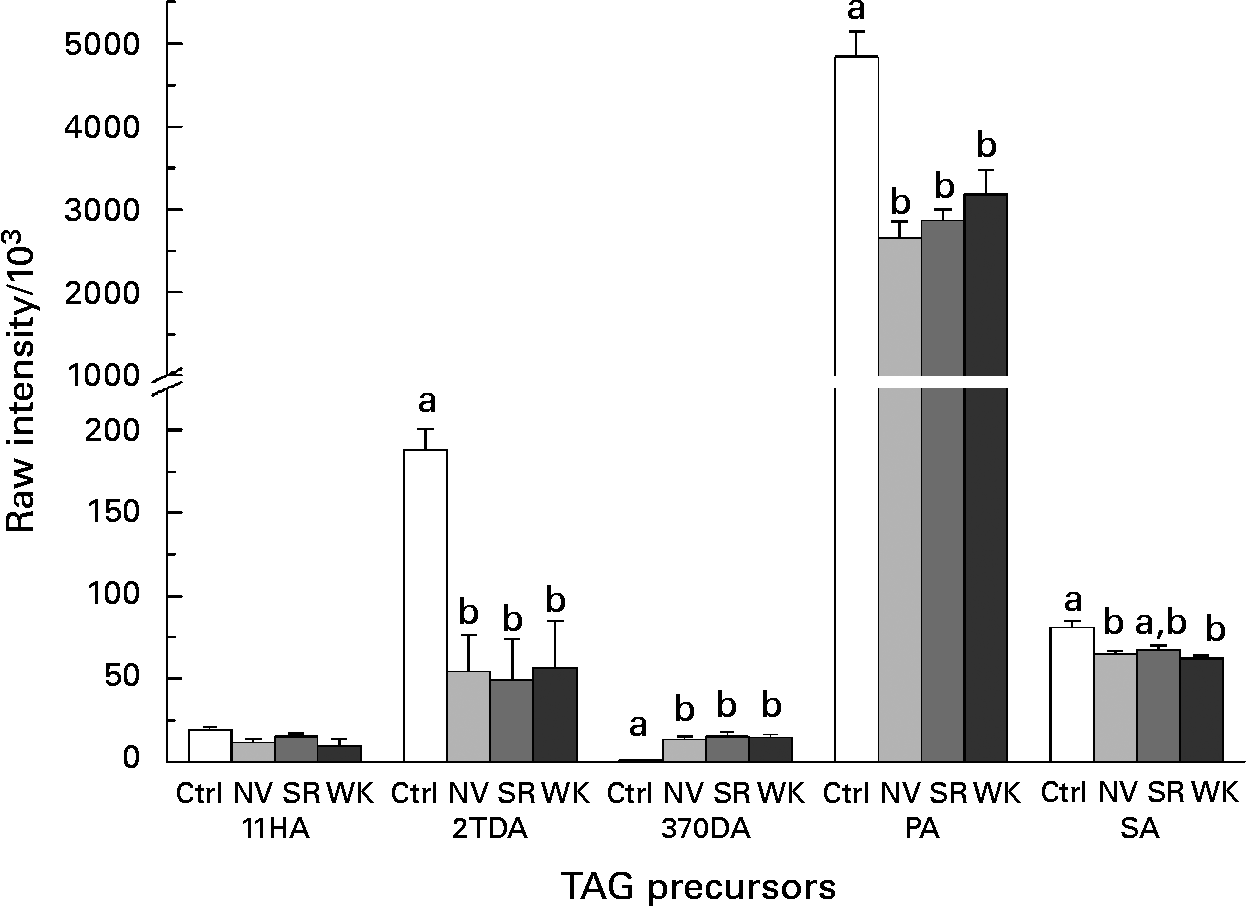
Fig. 2 Lipid metabolism in mammary gland tissue. Comparison of TAG precursors tentatively identified in mammary gland tissue. Values are means of at least nine animals per group (n 9–10), with standard errors represented by vertical bars. Metabolite intensity (relative concentration) was measured using liquid chromatography–time-of-flight MS and anlysed using Kruskal–Wallis one-way ANOVA. a,b Mean values with unlike letters were significantly different (P < 0·05). Ctrl, control; NV, navy bean-fed; SR, small red bean-fed; WK, white kidney bean-fed; 11HA, 11-hydroxy palmitic acid; 2TDA, 2-tetradecenoic acid; 37ODA, 3,7-octadecadiynoic acid; PA, palmitic acid; SA, stearic acid.
The effect of bean diet on n-6 fatty acids in the mammary gland
n-6 Fatty acids were compared between bean-fed and control animals. The compound tentatively identified as 18 : 3n-6 (γ-linolenic acid) was elevated in the three bean-fed groups (Kruskal–Wallis; P < 0·05) while adrenic acid (all-cis-7,10,13,16-docosatetraenoic acid) was reduced by more than 2-fold in all three bean-fed groups (Fig. 3). The reduction of adrenic acid was significantly different between control and NV bean-fed, SR bean-fed and WK bean-fed animals (Kruskal–Wallis; P < 0·05).

Fig. 3 n-6 Fatty acids tentatively identified in mammary gland tissue. Values are means of at least nine animals per group (n 9–10), with standard errors represented by vertical bars. Metabolite intensity (relative concentration) was measured using liquid chromatography–time-of-flight MS and analysed using Kruskal–Wallis one-way ANOVA. a,b Mean values with unlike letters were significantly different (P < 0·05). Ctrl, control; NV, navy bean-fed; SR, small red bean-fed; WK, white kidney bean-fed; LA, 18 : 3n-6 (γ-linolenic acid); AA, adrenic acid.
The effect of bean diet on diacylglycerol levels in the mammary gland
A total of five ions tentatively identified as diacylglycerols were reduced by the inclusion of dry edible beans in the diet (Fig. 4). Relative concentration of all five diacylglycerols was statistically different between each of the bean-fed groups and the control-fed animals (Kruskal–Wallis; P < 0·05). None of these molecules was statistically different between SR and NV bean-fed groups and only the diacylglycerols with masses of 586·4595 and 720·5706 Da were statistically different between WK and SR bean-fed (P < 0·05) and WK and NV bean-fed (P < 0·05) groups.
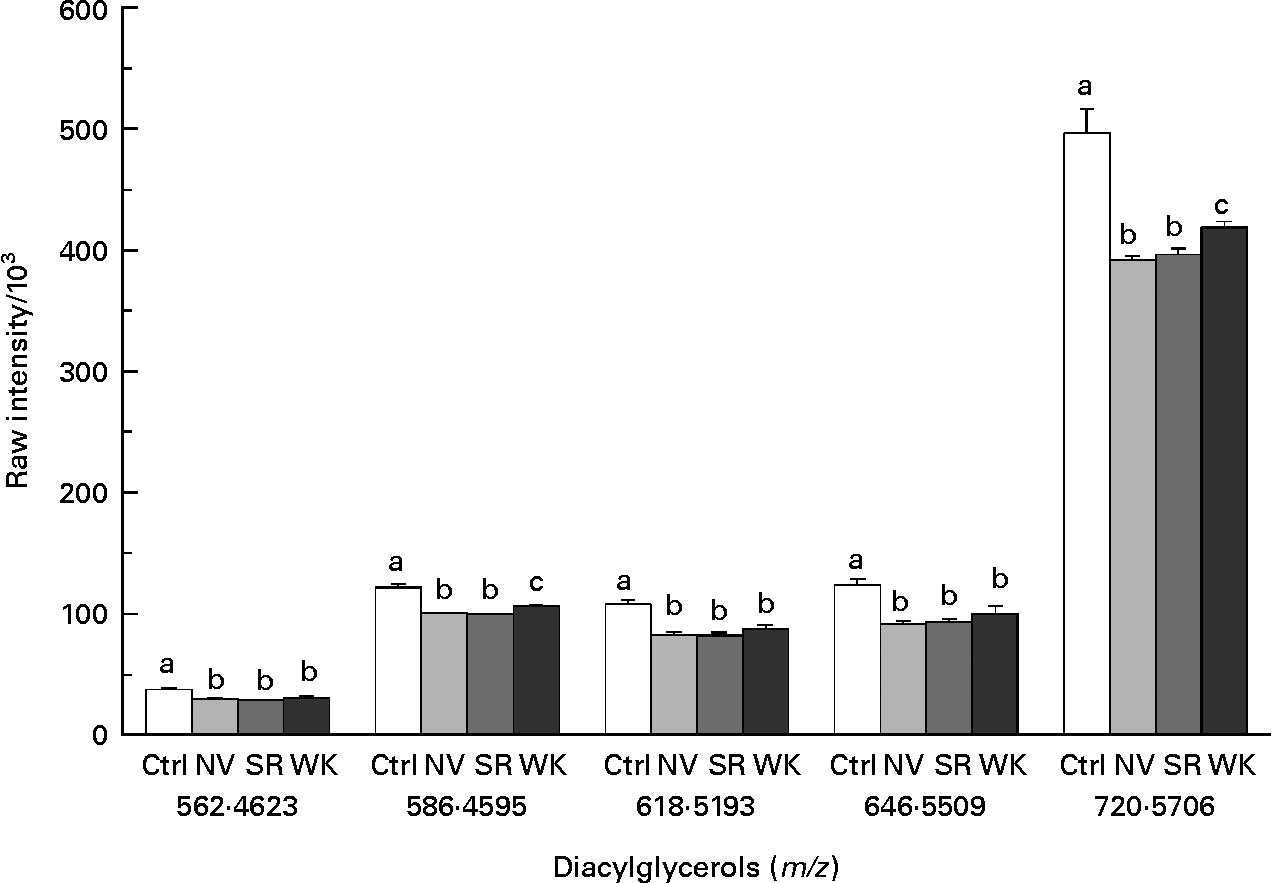
Fig. 4 Reduction of diacylglycerols in mammary gland as a result of bean feeding. The mass of each tentatively identified diacylglycerol is given below each set of four treatment groups. Values are means of at least nine animals per group (n 9–10), with standard errors represented by vertical bars. Metabolite intensity (relative concentration) was measured using liquid chromatography–time-of-flight MS and analysed using Kruskal–Wallis one-way ANOVA. a,b,c Mean values with unlike letters were significantly different (P < 0·05). Ctrl, control; NV, navy bean-fed; SR, small red bean-fed; WK, white kidney bean-fed.
The effect of bean diets on the mammary carcinoma metabolite profile
As was the case in the mammary gland tissue, distinct clustering based on bean type is evident in the scores plot generated following unsupervised PCA in both positive (Fig. 5(A)) and negative (Fig. 5(B)) ionisation modes with the first component explaining the difference between the bean-fed groups and the control-fed group in both cases. The first three components in positive mode explain 95·18, 2·09 and 1·63 % of the total variance in the dataset whereas the first three components in negative mode explain 61·02, 23·1 and 8·97 % of the total variance. Distinct clustering of the samples based on bean type is shown by the PCA scores plots of both positive and negative ionisation modes. Of the 2119 features detected in positive ionisation mode, 379 were statistically different between the bean groups and control group (one-way ANOVA; P < 0·05), with 327 higher in the bean-fed animals and just fifty-two higher in the control group. In contrast, of the 838 features detected in negative ionisation mode, all fifty-nine found to be statistically different between the bean-fed and control animals were higher in the control group. A total of twenty-eight features were tentatively identified after submission to MassTRIX using R. norvegicus (brown rat) as the model organism (Table 2).

Fig. 5 Principal component analysis (PCA) of the effect of bean diet on tumour tissue. (A) Electrospray ionisation (ESI)-positive, (B) ESI-negative. PCA shows a separate clustering based on control (![]() ), white kidney (
), white kidney (![]() ), small red (
), small red (![]() ) and navy (
) and navy (![]() ) bean diets.
) bean diets.
Table 2 Metabolites tentatively identified in mammary carcinomas*
RT, retention time; ESI+, electrospray ionisation positive mode; ESI − , electrospray ionisation negative mode.
* List based on MassTRIX query of Kyoto Encyclopedia of Genes and Genomes (KEGG).
† Indicates feature is increased in bean-fed animals ( ↑ ) or decreased in bean-fed animals ( ↓ ) relative to the control group.
‡ Metabolite type assigned based on KEGG search using MassTRIX. Total number of masses searched = 350, metabolite types were identified = 28/350 or 8·0 %, non-identified metabolite types = 322/350 or 92·0 % (not shown).
The effect of bean diet on fatty acids and fatty aldehyde compounds in mammary carcinomas
Fatty acyl compounds were tentatively identified as features differentially regulated with the inclusion of beans in the diet depending upon the subclass of molecule. Figure 6 illustrates this for (A) methyl branched fatty acids, (B) straight-chain fatty acids, (C) oxo fatty acids and (D) fatty aldehydes. Compounds with masses corresponding to methyl branched fatty acids (2,14-dimethyl hexanoic acid and isopalmitic acid) and the fatty aldehyde (9-octadecenal) were reduced in the bean-fed groups (P < 0·05). In addition, 3,7,11-trimethyl-docecanoic acid (methyl branched fatty acid) and the fatty aldehyde 2-tetradecenal were not detected in the bean-fed groups. In contrast, molecules putatively identified as straight-chain fatty acids (3-capryl propionic acid, 8-oxo-nonanoic acid) and the oxo fatty acid caproic acid were not detected in the control group. The molecule tentatively identified as octanoic acid was significantly increased in all three bean groups (Kruskal–Wallis; P < 0·05). Additionally, isopalmitic acid, 2,14-dimethyl hexanoic acid, caproic acid, octanoic acid, 3-capryl propionic acid, 8-oxo-nonanoic acid and 9-octadecenal were statistically different between WK and NV bean-fed groups as well as between WK and SR bean-fed animals (Kruskal–Wallis; P < 0·05). Interestingly, only isopalmitic acid was statistically different between the NV and SR bean-fed groups (Kruskal–Wallis; P < 0·05).

Fig. 6 Effect of bean diet on fatty acyl compounds in mammary carcinomas. (A) Methyl branched fatty acids, (B) straight-chain fatty acids, (C) oxo fatty acids, (D) fatty aldehydes. Data are shown as fold change relative to control. Values are means of at least nine animals per group (n 9–10), with standard errors represented by vertical bars. Metabolite intensity (relative concentration) was measured using liquid chromatography–time-of-flight MS and analysed using Kruskal–Wallis one-way ANOVA. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05). n.d., Not detected; Ctrl, control; NV, navy bean-fed; SR, small red bean-fed; WK, white kidney bean-fed; DHA, 2,14-dimethyl hexanoic acid; IPA, isopalmitic acid; TDA, 3,7,11-trimethyl docecanoic acid; CA, caproic acid; OA, octanoic acid; CPA, 3-capryl propionic acid; ONA, 8-oxo-nonanoic acid; TA, 2-tradecenal; OD, 9-octadecenal.
Bean diet-induced changes in tumour prostaglandins
Ions putatively identified as prostaglandins, in particular the E series, were up-regulated by bean diets. The increase in PGE3 was statistically significant (Kruskal–Wallis; P < 0·05) when comparing the three bean-fed groups individually with the control. This particular prostaglandin mimics the cancer-prevention activity of the bean races, high cancer-prevention activity correlates with the larger increase in PGE3 (WK, 88 521·667 (sem 7680·379)), intermediate cancer-prevention activity correlates with an intermediate level of PGE3 (SR, 91 011·714 (sem 5906·439)) and the lowest cancer-prevention activity correlates with the lowest PGE3 level (NV, 101 044·769 (sem 6969·341)). Interestingly, PGE3 was significantly different between the WK and SR (Kruskal–Wallis, P < 0·05) as well as between the WK and NV genotypes (Kruskal–Wallis; P < 0·05) but not between the NV and SR genotypes (Kruskal–Wallis; P = 0·995) (data not shown).
Discussion
There is currently a resurgence of interest in encouraging increased consumption of pulses such as dry beans. However, newly released dietary guidelines from the US Department of Agriculture do not support this trend, in part because Americans have failed to achieve the previously recommended amounts(25). Rather, the new guidelines suggest that pulses be consumed in reduced amounts in comparison with previous guidelines(26). While this seems surprising at first glance, a more careful review of the evidence reveals a body of literature that is supportive of pulse consumption but that fails to make a compelling case for their inclusion in the diet, in part due to known barriers to eating beans and to a lack of solid mechanistic data that account for health benefits(Reference Bennink and Rondini9, Reference Duane16–Reference Guillon and Champ18). In the present paper, we used metabolomic profiling as a novel approach to test the global hypothesis that dry bean consumption promotes health through the regulation of lipid metabolism(Reference Gibney, Walsh and Brennan27). While evidence for this hypothesis has emerged from several sources, to our knowledge this represents the first report of the modulation of lipid metabolism by dry beans using an ‘omics’ approach. As discussed in the following paragraphs, the data obtained clearly indicate that lipid metabolism is modified in both mammary gland and mammary carcinomas of bean-fed rats relative to rats fed a control (bean-free) diet, and that argue additional experiments are warranted to identify specific gene targets that account for the observed effects on lipid metabolism. The data reported also are consistent with variation among bean genotypes in the traits that alter lipid metabolism and thus indicate the potential for plant scientists to improve dry beans through classical approaches used in plant breeding and selection.
Metabolomics is a high-throughput screening tool that lends itself to hypothesis generation at the level of cells or tissues(Reference Weckwerth28). While the metabolomics field is still limited in its application to the identification of natural products and bioactive food components in plant materials because of the libraries that are currently available for ion identification, the compound libraries for mammalian metabolites are sufficiently developed to permit tentative identification and association with the metabolic pathways(Reference Tohge and Fernie29). Accordingly, Tables 1 and 2 contain lists of ions to which identities were assigned; these identities are tentative because of the need to analyse validated standards, assess percentage recovery, and perform standard curve experiments for quantitative assessments using the conditions of chromatography and mass analysis defined in the Methods section. Validation of compound identification is considered a next step in this programme of research. Nonetheless, the qualitative data shown in Tables 1 and 2 clearly indicate that dry bean consumption has effects on many lipid metabolites and therefore the cellular pathways responsible for their synthesis, interconversion and/or degradation.
Many approaches can be taken to analyse and interpret metabolomics data and although there are clearly on-going efforts to create a uniform framework for analysis(Reference Kind and Fiehn30, Reference Scalbert, Brennan and Fiehn31), a standardised method does not currently exist. Our protocol was: (1) to filter data according to criteria that permitted generation of a ‘high-quality’ ion database consisting of ion masses and retention times; (2) to subject that list to statistical analysis to generate a sublist of ions that differed significantly between treatment groups; (3) to submit the ion list from point 2 to unsupervised PCA, to ask whether treatment with dietary beans affected the metabolome of the mammary gland (Fig. 1) or the mammary carcinomas (Fig. 5) that occurred in control v. bean-fed rats; and (4) to submit the list of masses used in PCA to MassTRIX for tentative ion identification and association with metabolic pathways (Tables 1 and 2). As noted in the Results section, separation between control and bean-fed rats was observed in both mammary gland and mammary carcinomas. This finding represents a critical proof-of-concept that eating a dry bean-containing diet exerts effects on mammalian cellular metabolism in two peripheral tissues with markedly different cellular composition. Mammary gland is a tissue that is primarily comprised of adipocytes; however, mammary carcinomas are primarily comprised of epithelial cells. Both tissues types have a heterogeneous mixture of cell types, but mammary gland is physiologically adapted for milk production; however, mammary carcinomas are pathologies, the cells of which display altered metabolic characteristics as outlined in Wood et al. (Reference Wood, Parsons and Jones32).
With the proof-in-principle evidence in hand, the same mass lists used in the PCA analyses were submitted to MassTRIX(Reference Suhre and Schmitt-Kopplin33). This analysis tool links ion masses to a database of candidate compound identities also linked to the KEGG metabolic pathways. MassTRIX analysis was first completed for mammary gland and the resulting list of hits for the masses entered is provided in Table 1. Most noteworthy was the prominent identification of lipid metabolites as being up- or down-regulated in the bean-fed diet groups relative to the control diet-fed group (Figs. 2–4). KEGG links were identified to lipid biosynthesis, steroid biosynthesis and intermediary metabolism of lipids. This finding suggests that bioactive components of beans are likely to be affecting key regulatory elements in lipid metabolism and supports evaluating the effects of dry bean consumption on enzyme loci such as acetyl CoA carboxylase, fatty acid synthase, and 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase, key regulators of lipid metabolism. It should be noted that other lipid-metabolising enzymes are also likely to be involved.
With evidence that mammary gland lipid metabolism was affected by bean consumption, the metabolomics database for mammary carcinomas was then submitted to MassTRIX despite the fact that a carcinoma in the bean-treated group reflected the failure of beans to inhibit carcinoma development. The analysis of carcinomas in this model system has been useful in previous experiments in garnering clues about mechanisms of effect. The masses for which tentative compound identification were provided by MassTRIX are shown in Table 2. As with normal mammary gland, lipid metabolism was highly enriched in the pathways identified. However, there were differences in the nature of the pathways implicated. For example, diacylglycerol was lower in the normal mammary glands of bean-fed rats; alterations in prostaglandin metabolism were implied in the mammary carcinomas. This is likely to be informative since differential regulation of eicosanoid synthesis and degradation is known to be involved in the genesis and progression of cancer(Reference Wang and Dubois34).
One goal of the present study was to develop hypotheses about mechanisms that could account for the inhibition of mammary carcinogenesis that we have previously reported(Reference Thompson, Brick and McGinley14, Reference Thompson, Thompson and Brick15). The results of the analyses of normal mammary gland and mammary carcinomas provided the rationale for two hypotheses. The mammary gland data are consistent with the hypothesis that bean feeding down-regulates formation of diacylglycerols and related intracellular messengers which affect signalling by either protein kinase C or the nutrient-sensing master control protein, mammalian target of rapamycin (mTOR)(Reference Laplante and Sabatini35, Reference Sakane, Imai and Kai36). Negative regulation of the pathways of these proteins and components has been reported to be a mechanism of cancer prevention, whereas misregulation of mTOR has been implicated in the development of cancer. Our second hypothesis is based on the data from the mammary carcinomas that we have reported to be markedly smaller in bean-fed relative to control-fed rats. We hypothesise that bean consumption modulates eicosanoid metabolism in mammary carcinomas and that this results in lower tumour mass by changing the ratios of eicosanoids within the tumour. From the viewpoint of metabolic regulation, this implicates involvement of cyclo-oxygenase-2, the rate-limiting step in eicosanoid degradation which is catalysed by prostaglandin dehydrogenase, a catabolic enzyme that serves a potent tumour suppressor function(Reference Muller-Decker and Furstenberger37–Reference Wolf, O'Kelly and Rubinek39). In addition to these ideas, it is important to consider that rapidly dividing tumour cells require robust de novo synthesis of lipids as part of the metabolic reprogramming that is essential for the development and progression of cancer(Reference Jones and Thompson40, Reference Kaelin and Thompson41). Given that bean consumption appears to target lipid metabolism, investigation of the effects on the reprogramming of metabolism that occurs in tumours is warranted since bean consumption could serve to enhance the efficacy of cancer therapy as well as play a role in cancer prevention.
Each type of bean that was studied is representative of a distinct dry bean market class. Bean market classes share comparable seed size, shape, colour and texture but are actually collections of similar genotypes within specific gene pools(Reference Kami, Velasquez and Debouck42). Market classes arose during domestication as a result of human selection for plant morphology and seed characteristics leading to modern-day differences among seed types. Bean market classes are further organised by race and most broadly by centre of domestication (COD)(Reference Singh43, Reference Singh, Gepts and Debouck44). The COD of an agricultural crop denotes the location where domestication took place. The SR bean is a market class in the Middle American COD, race – Durango, the NV bean is also a market class in the Middle American COD, but race – Mesoamerican, and the WK market class is from the Andean COD, race – Nueva Granada. Beans from these races account for more than 80 % of worldwide common bean production(Reference Singh, Gepts and Debouck44). Knowing that these bean types represent distinct genetic heritage, an additional question addressed was whether these genetic distinctions translated into differential effects on tissue metabolomes. The PCA analyses shown in Figs. 1 and 4 provide evidence that these genotypically distinct dry bean market classes caused distinguishable differences in the mammary gland and the mammary carcinoma metabolomes, specifically in lipid metabolites (Tables 1–2). While this finding has many implications, a practical value is that these differences will serve to provide well-matched positive and negative controls for detailed analyses to determine how dry bean components regulate genes that control lipid biosynthesis and catabolism.
In summary, mounting evidence indicates that the decline in dry bean consumption that has occurred during global industrialisation and economic development may have associations with the rise in chronic disease rates that have accompanied the pandemic of obesity. The effects that dry beans may exert on chronic disease mechanisms are diverse and have not yet been extensively investigated. The evidence presented here identifies lipid metabolism as a critical target of bean bioactive food components for future studies with a focus on determining how dry bean consumption affects key regulators of lipid biosynthesis, interconversion and degradation.
Acknowledgements
The present study was supported in part by USAID grant no. REE-A-00-03-00094-00, the Bean Health Alliance, the American Institute for Cancer Research grant no. 08A032, and the US Department of Agriculture National Institute of Food and Agricultural Agriculture and Food Research Initiative Project no. 2009-01929. The authors thank Steven Fischer, Agilent Technologies for his assistance with the analytical work, Mark Brick for editorial assistance, and Erica Danielle, Vanessa Fitzgerald, Elizabeth Neil, and Jennifer Sells for their excellent technical assistance. M. M. M. conducted the metabolomics analyses including data evaluation. J. N. M. participated in various aspects of study implementation and data evaluation and interpretation. H. J. T. designed the carcinogenesis study and provided oversight of all aspects of the experiment. All authors participated in writing the manuscript. There are no conflicts of interest.










