Diabetic nephropathy is one of the most serious long-term complications in terms of morbidity and mortality in diabetic patients(Reference Soman and Soman1). Although hyperglycaemia is considered as the prime metabolic aberrance, numerous other mechanisms have also been suggested for the development of diabetic nephropathy(Reference Schrijvers, De Vriese and Flyvbjerg2). Many studies have demonstrated that abnormal inflammatory responses including macrophage infiltration and overexpression of pro-inflammatory cytokines are present in the diabetic kidney(Reference Chow, Ozols and Nikolic-Paterson3). Moreover, hyperglycaemia-induced generation of reactive oxygen species (ROS) is associated with inflammation by activating transcription factors such as NF-κB(Reference Ha, Yu and Choi4). Activation of NF-κB increases TNF-α, IL-1β, transforming growth factor-β1 and vascular endothelial growth factor. These pro-inflammatory cytokines and growth factors involved in the direct damage of kidneys, glomerulosclerosis and angiogenesis thus stimulate the development of diabetic nephropathy. Indeed, many studies have revealed that lipid peroxidation accelerated by oxidative stress is increased in diabetic renal glomerular lesions(Reference Horie, Miyata and Maeda5). Although the involvement of inflammatory processes in the development and progression of diabetic nephropathy has been recognised, intervention for inflammation in the diabetic kidney is practically unknown.
Some studies have shown that diabetic patients have a significant deficiency of antioxidant activity, which may cause high vulnerability on oxidative stress and the development of diabetic complications. Plasma antioxidant status, including total radical-trapping antioxidant capacity and the levels of uric acid, vitamin A, vitamin C or vitamin E, was decreased in diabetes(Reference Maxwell, Thomason and Sandler6–Reference Santini, Marra and Giardina9). In previous studies, nutrients such as α-tocopherol, taurine, N-acetylcysteine (NAC) or α-lipoic acid have improved glucose disposal, suppressed protein kinase C activation and decreased oxidative stress, as well as prevented the formation of advanced glycation end products and inhibited the levels of glycated proteins(Reference Studer, Craven and DeRubertis10–Reference Bierhaus, Chevion and Chevion13). In addition, dietary curcuminoids have a protective role through the scavenging activity of ROS produced by hyperglycaemia, and they also induce antioxidative enzymes(Reference Osawa and Kato14). Supplementation of NAC, a precursor of glutathione (GSH), has suppressed NF-κB activation properly and decreased hyperglycaemia(Reference Ho, Chen and Bray15). Vitamin C and GSH treatment have significantly decreased oxidative stress and preserved renal function in diabetic rats(Reference Lee, Lee and Hong16–Reference Nakhoul, Miller-Lotan and Awad18). Many studies have supported the role of antioxidants in preventing the development of diabetic nephropathy(Reference Craven, DeRubertis and Kagan19–Reference Packer, Witt and Tritschler22). However, single antioxidant nutrients were utilised, and their effects were limited. Furthermore, little research has been conducted using dietary antioxidant cocktails as modulators of oxidative stress and inflammatory response in diabetic nephropathy. Thus, the present study was conducted to determine and compare the individual effects of a single dietary supplementation of antioxidants with the synergetic effects of antioxidants interacting with each other, vitamin C, vitamin E and/or NAC, on diabetic nephropathy through modulation of the inflammatory response. We used kidneys of diabetic mice induced by alloxan that selectively devastates the insulin-producing β-cells in the pancreas(Reference Ramadan, El-Beih and Abd El-Ghffar23) to examine the effects of dietary antioxidant supplementation on renal inflammation and temporal expression levels of Cu-Zn superoxide dismutase (SOD), Mn-SOD, haeme oxygenase-1, phosphorylated IκBα (pIκBα), inducible NO synthase (iNOS), cyclo-oxygenase-2 (COX-2) and C-reactive protein (CRP) proteins.
Experimental methods
Animals induced with diabetes mellitus
Female ICR (CD-1) mice (4 weeks old) were purchased from Daehan Biolink Company, Limited (Incheon, Republic of Korea). Mice were individually housed in polycarbonate cages with wire tops in a room maintained at 22 ± 1°C and 50 ± 1 % humidity on a 12 h light–12 h dark cycle with free access to water and a chow diet for 1 week. Diabetes was induced by alloxan monohydrate (180 mg/kg body weight, intraperitoneal injection; Sigma-Aldrich, St Louis, MO, USA) in a saline solution. Non-diabetic control mice were injected with saline. After 5 d, the induction of diabetes was confirmed by measuring fasting blood glucose levels. Body weights and blood glucose levels were measured once a week. Glucose concentration was measured by Accu-Chek sensor (Roche Diagnostics Company Limited, Seoul, Republic of Korea) in blood obtained from tail veins at the same time to minimise the effect of diurnal fluctuation. All mice were used in accordance with animal protocols approved by the Kyung Hee University Institutional Animal Care and Use Committee.
Experimental diets
Mice with a fasting blood glucose level ≥ 2500 mg/l were used for the study. Mice were divided into five groups that were fed different antioxidant supplementation: (1) group 1 (CON) – non-diabetic control mice fed an AIN 93G Rodent purified diet (Research Diets, New Brunswick, NJ, USA), (2) group 2 (DM) – diabetic mice fed an AIN 93G Rodent purified diet, (3) group 3 (NAC) – diabetic mice fed a 0·5 g NAC/100 g diet (Sigma grade, ≥ 99 %; Sigma-Aldrich), (4) group 4 (VCE) – diabetic mice fed a 0·5 g vitamin C/100 g diet (l-ascorbic acid, ≥ 99·0 %, crystalline; Sigma-Aldrich) and 0·5 g vitamin E/100 g diet ((+/ − ) α-tocopherol, synthetic, ≥ 96 %; Sigma-Aldrich), (5) group 5 (Comb) – diabetic mice fed a 0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet. All mice were fed the treatment diets and water ad libitum, throughout the experimental period (8 weeks).
Sample collection and preparation
Mice were killed under light diethyl ether anaesthesia. Blood samples were collected by cardiac puncture into heparin-containing tubes and centrifuged at 3000 rpm for 10 min at 4°C. The kidneys were excised and rinsed in saline and stored at − 80°C until processed.
Measurement of lipid peroxidation product in tissue
Kidney thiobarbituric acid-reacting substance (TBARS) levels were measured as an index of lipid peroxidation. This process was assayed according to the method of Ohkawa et al. (Reference Ohkawa, Ohishi and Yagi24). Briefly, 0·1 g kidney was homogenated in a 0·15 m-KCl buffer. Samples (200 μl) were added to 200 μl of 8·1 % SDS and placed in room temperature for 10 min, and 3 ml of a 20 % acetic acid–0·8 % thiobarbituric acid mixture along with 600 μl of distilled water were added. The mixture was heated at 95°C for 1 h in a boiling water-bath. After cooling, 1 ml distilled water and 5 ml mixture of n-butanol and pyridine were added and shaken vigorously. After the mixture was centrifuged at 4000 rpm for 10 min, the absorbance of the supernatant was measured at 532 nm using 1,1,3,3-tetramethoxypropane (Sigma-Aldrich) as an external criterion.
Renal function test
Renal functions in diabetic kidneys were assessed by measuring the blood urea N (AM 165; Asan Pharmaceutical Company Limited, Seoul, Republic of Korea) and plasma creatinine levels (DICT-500; Bioassay Systems, Inc., Hayward, CA, USA) using commercial kits, according to the manufacturer's manual. Reaction end products were read immediately at 580 nm (blood urea N) and 510 nm (creatinine) using an ELISA reader.
Western blotting assay
Kidney (0·1 g) was homogenised in a lysis buffer (containing Trizma base, NaCl, 10 % NP40, 10 % Na-dedoxycholate, 100 mm-EDTA and 10 % SDS) with protease inhibitor (1:200; Sigma-Aldrich) and centrifuged at 14 000 rpm for 30 min. The sample (60 μg protein) was separated on a 10 % SDS PAGE and then transferred to membranes. After blocking for 1 h in 5 % skimmed milk, the membranes were incubated with specific primary antibodies against CuZn-SOD (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), Mn-SOD (1:5000; Stressgen, Victoria, BC, Canada), HO-1 (1:1500; Stressgen), pIκBα (1:200; Santa Cruz Biotechnology), iNOS (1:2000; Stressgen), COX-2 (1:250; Transduction Laboratories, Lexington, KY, USA), CRP (1:400; Abcam, Cambridge, MA, USA) and β-actin (1:800; Santa Cruz Biotechnology) for overnight at 4°C, and then incubated with secondary antibodies for 1 h at room temperature. The blots were detected by enhanced chemiluminescence using an enhanced chemiluminescence solution and measured by the ImageJ program (National Institutes of Health, Bethesda, MD, USA. The target protein expression levels were normalised by β-actin protein expression in each sample.
Statistical analysis
All values are expressed as means with their standard errors. Data were analysed by one-way ANOVA using SPSS version 12.0 software (SPSS, Inc., Chicago, IL, USA), and then differences among means were analysed using Duncan's test. The relationships among blood glucose levels, TBARS, blood urea N and creatinine levels were evaluated by Pearson's correlation coefficients. For all tests, differences were considered significant at P values of < 0·05, < 0·01 and < 0·001.
Results
Body weight
As shown in Fig. 1, the CON group revealed a significantly higher body-weight gain than all diabetic groups (DM, NAC, VCE and Comb) throughout the experimental periods, but the VCE and Comb groups improved the body-weight gain compared with the DM group by 123 and 125 % (P < 0·05), respectively.

Fig. 1 Effects of dietary antioxidant supplementation on body-weight change in alloxan-induced diabetic mice. CON (control mice, ![]() ), DM (diabetic control mice,
), DM (diabetic control mice, ![]() ), NAC (N-acetylcysteine,
), NAC (N-acetylcysteine, ![]() ) (0·5 g NAC/100 g diet-supplemented diabetic mice), VCE (
) (0·5 g NAC/100 g diet-supplemented diabetic mice), VCE (![]() ) (0·5 g vitamin C/100 g diet and 0·5 g vitamin E/100 g diet-supplemented diabetic mice) and Comb (
) (0·5 g vitamin C/100 g diet and 0·5 g vitamin E/100 g diet-supplemented diabetic mice) and Comb (![]() ) (0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet-supplemented diabetic mice). Values are means, with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
) (0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet-supplemented diabetic mice). Values are means, with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
Blood glucose levels
All diabetic groups regardless of dietary antioxidant treatment showed significantly higher blood glucose levels than the CON group after alloxan was injected (P < 0·05; Fig. 2). After 3 weeks of diet treatment, blood glucose levels of all diabetic mice supplemented with dietary antioxidants (NAC, VCE, and Comb) were significantly lower compared with that of the DM group by 77, 76 and 62 % (P < 0·05), respectively. Furthermore, in the Comb group, the blood glucose level was significantly lower after 4 weeks of dietary antioxidant treatment compared with those of the NAC and VCE groups (P < 0·05).

Fig. 2 Effects of dietary antioxidant supplementation on blood glucose levels in alloxan-induced diabetic mice. CON (control mice, ![]() ), DM (diabetic control mice,
), DM (diabetic control mice, ![]() ), NAC (N-acetylcysteine,
), NAC (N-acetylcysteine, ![]() ) (0·5 g NAC/100 g diet-supplemented diabetic mice), VCE (
) (0·5 g NAC/100 g diet-supplemented diabetic mice), VCE (![]() ) (0·5 g vitamin C/100 g diet and 0·5 g vitamin E/100 g diet-supplemented diabetic mice) and Comb (
) (0·5 g vitamin C/100 g diet and 0·5 g vitamin E/100 g diet-supplemented diabetic mice) and Comb (![]() ) (0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet-supplemented diabetic mice). Values are means, with their standard errors represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
) (0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet-supplemented diabetic mice). Values are means, with their standard errors represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
Lipid peroxidation levels in diabetic kidneys
The effects of dietary antioxidant supplementation on TBARS levels were evaluated in the kidneys. As shown in Table 1, the renal TBARS level of the DM group was significantly higher compared with that of the CON group (1·6 times, P < 0·05), but not in dietary antioxidant-supplemented diabetic mice.
Table 1 Levels of thiobarbituric-acid reacting substances (TBARS), blood urea nitrogen (BUN) and creatinine in control and diabetic mice with various dietary treatments for 8 weeks
(Mean values with their standard errors)
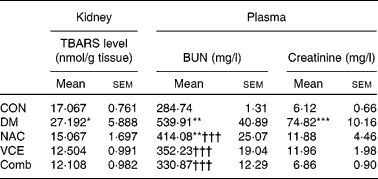
CON, non-diabetic control mice fed an AIN 93G Rodent purified diet; DM, diabetic mice fed an AIN 93G Rodent purified diet; NAC, diabetic mice fed a 0·5 g N-acetylcysteine/100 g diet; VCE, diabetic mice fed a 0·5 g vitamin C/100 g diet and 0·5 g vitamin E/100 g diet; Comb, diabetic mice fed a 0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet.
Mean values were significantly different from those of the CON group: *P < 0·05, **P < 0·01, ***P < 0·001.
Mean values were significantly different from those of the DM group: ††† P < 0·001.
Blood urea nitrogen and plasma creatinine levels
To determine the effect of dietary antioxidant supplementation on the protection of renal tissue against alloxan-induced diabetes, the levels of blood urea N and creatinine were determined. Experimental findings showed that the levels of blood urea N (two times) and creatinine (twelve times) in the DM group were significantly higher than those of the CON group (P < 0·01 and < 0·001). However, dietary antioxidant supplementation effectively ameliorated the increases in blood urea N and creatinine levels to nearly normal levels, respectively (Table 1).
Relationships among blood glucose levels, thiobarbituric acid-reacting substances, blood urea nitrogen and creatinine levels
Blood glucose levels in the last week of the experiment (after 8 weeks of the diet treatment) were positively correlated with TBARS, blood urea N and creatinine levels in CON and diabetic mice treated with different antioxidant-supplemented diets (P < 0·05; Table 2).
Table 2 Relationships among blood glucose levels, thiobarbituric acid-reacting substances (TBARS), blood urea nitrogen (BUN) and creatinine levels in control and diabetic mice with various dietary treatments for 8 weeks
(Pearson's correlation coefficients)

Values were significantly different: *P < 0·05, **P < 0·01.
† Blood glucose levels in the last week of the experiment (after 8 weeks of the diet treatment).
Relationships among thiobarbituric acid-reacting substances, blood urea nitrogen and creatinine levels
TBARS levels were positively correlated with blood urea N and creatinine levels in CON and diabetic mice treated with different antioxidant-supplemented diets (P < 0·05; Table 3).
Table 3 Relationships among thiobarbituric acid-reacting substances (TBARS), blood urea nitrogen (BUN) and creatinine levels in control and diabetic mice with various dietary treatments for 8 weeks
(Pearson's correlation coefficients)

*P < 0·05, **P < 0·01.
Oxidative stress- and inflammatory response-related protein expression in diabetic kidneys
To establish the effects of dietary antioxidant supplementation on renal inflammation in diabetic mice, we investigated the expression levels of oxidative stress- and inflammatory response-related proteins in mouse kidneys using Western blotting. The expression levels of CuZn-SOD are shown in Fig. 3. We found a higher protein expression of CuZn-SOD in the kidneys of the DM, NAC and VCE groups than in those of the CON group (P < 0·05) but in the Comb group, the levels of CuZn-SOD returned to a normal state. However, Mn-SOD protein expression did not significantly differ in all experimental groups (data not shown). HO-1 protein expression in the kidneys was considerably down-regulated in the DM group compared with the CON group (P < 0·05), but dietary antioxidant supplementation inhibited from decreasing HO-1 protein expression to a level consistent with the CON group (Fig. 4). The level of pIκBα protein was significantly higher in the kidneys of the DM group compared with that of the CON group (P < 0·05; Fig. 5). Dietary antioxidant-supplemented diabetic mice significantly reduced the pIκBα protein levels, and notably, the antioxidant cocktail-supplemented VCE and Comb groups decreased to a greater amount the pIκBα protein level than the single antioxidant-supplemented NAC group (P < 0·05). While there was no difference in the expression levels of iNOS among the CON, DM, NAC and VCE groups, the Comb group significantly diminished the iNOS protein levels (P < 0·05; Fig. 6). As shown in Fig. 7, the expression levels of COX-2 protein were significantly higher in the DM group compared with those of the CON group (P < 0·05). On the other hand, the COX-2 levels were remarkably reduced in dietary antioxidant-supplemented diabetic mice (P < 0·05). The expression levels of CRP were lower in the Comb group compared with those in the DM group (P < 0·05; Fig. 8).

Fig. 3 Effects of dietary antioxidant supplementation on protein expression levels of Cu-Zn superoxide dismutase (CuZn-SOD) in alloxan-induced diabetic kidney. CON (control mice), DM (diabetic control mice), NAC (N-acetylcysteine, 0·5 g NAC/100 g diet-supplemented diabetic mice), VCE (0·5 g vitamin C/100 g diet and 0·5 g vitamin E/100 g diet-supplemented diabetic mice) and Comb (0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet-supplemented diabetic mice). Values are means, with their standard errors represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).

Fig. 4 Effects of dietary antioxidant supplementation on protein expression levels of haeme oxygenase-1 (HO-1) in alloxan-induced diabetic kidney. CON (control mice), DM (diabetic control mice), NAC (N-acetylcysteine, 0·5 g NAC/100 g diet-supplemented diabetic mice), VCE (0·5 g vitamin C/100 g diet and 0·5 g vitamin E/100 g diet-supplemented diabetic mice) and Comb (0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet-supplemented diabetic mice). Values are means, with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05).

Fig. 5 Effects of dietary antioxidant supplementation on protein expression levels of phosphorylated IκBα (pIκBα) in alloxan-induced diabetic kidney. CON (control mice), DM (diabetic control mice), NAC (N-acetylcysteine, 0·5 g NAC/100 g diet-supplemented diabetic mice), VCE (0·5 g vitamin C/100 g diet and 0·5 g vitamin E/100 g diet-supplemented diabetic mice), Comb (0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet-supplemented diabetic mice). Values are means, with their standard errors represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).

Fig. 6 Effects of dietary antioxidant supplementation on protein expression levels of inducible NO synthase (iNOS) in alloxan-induced diabetic kidney. CON (control mice), DM (diabetic control mice), NAC (N-acetylcysteine, 0·5 g NAC/100 g diet-supplemented diabetic mice), VCE (0·5 g vitamin C/100 g diet and 0·5 g vitamin E/100 g diet-supplemented diabetic mice) and Comb (0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet-supplemented diabetic mice). Values are means, with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05).

Fig. 7 Effects of dietary antioxidant supplementation on protein expression levels of cyclo-oxygenase-2 (COX-2) in alloxan-induced diabetic kidney. CON (control mice), DM (diabetic control mice), NAC (N-acetylcysteine, 0·5 g NAC/100 g diet-supplemented diabetic mice), VCE (0·5 g vitamin C/100 g diet and 0·5 g vitamin E/100 g diet-supplemented diabetic mice) and Comb (0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet-supplemented diabetic mice). Values are means, with their standard errors represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).

Fig. 8 Effects of dietary antioxidant supplementation on protein expression levels of C-reactive protein (CRP) in alloxan-induced diabetic kidney. CON (control mice), DM (diabetic control mice), NAC (N-acetylcysteine, 0·5 g NAC/100 g diet-supplemented diabetic mice), VCE (0·5 g vitamin C/100 g diet and 0·5 g vitamin E/100 g diet-supplemented diabetic mice) and Comb (0·5 g vitamin C/100 g diet, 0·5 g vitamin E/100 g diet and 0·5 g NAC/100 g diet-supplemented diabetic mice). Values are means, with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05).
Discussion
In these experiments, we investigated the effects of dietary antioxidant supplementation on molecular events and kidney function in diabetic nephropathy induced by alloxan that damages pancreatic β cells(Reference Ramadan, El-Beih and Abd El-Ghffar23). Our data demonstrated that lipid peroxidation in kidneys and renal function were improved by dietary antioxidant supplementation. Moreover, antioxidant cocktail treatment attenuated not only blood glucose levels but also specific markers of the inflammatory response, including protein levels of the pIκBα, inflammation-mediated enzymes, iNOS and COX-2, and the acute-phase protein, CRP.
In the present study, the body weights of the VCE and Comb groups, rather than the NAC group, were higher compared with that of the DM group. A previous study has shown that the body weights of diabetic rats supplemented with vitamin C or vitamin E did not differ from those of untreated diabetic rats, suggesting that supplementation with an antioxidant cocktail may have a positive anabolic effect by the regulation of blood glucose levels(Reference Kedziora-Kornatowska, Szram and Kornatowski25). Previous studies have shown that vitamin C, vitamin E or NAC significantly decreased the TBARS levels(Reference Park, Park and Lim26–Reference Rhoden, Lawrence and Godleski28). Moreover, our previous study showed that a short-term as well as a long-term dietary antioxidant cocktail treatment decreased TBARS levels in the diabetic kidneys(Reference Cay, Naziroglu and Simsek29). However, a long-term dietary antioxidant supplementation for diabetic mice in the present study returned the levels of TBARS towards the controls' levels, which suggests that long-term antioxidant treatment is more effective in reducing oxidative stress. We demonstrated that increased renal oxidative stress represented by TBARS is positively correlated with increased renal dysfunction. Increased blood urea N and plasma creatinine in diabetic rats indicates progressive renal damage(Reference Rhoden, Lawrence and Godleski28, Reference Renno, Abdeen and Alkhalaf30). It has been previously reported that diabetic rats had a decreased renal function that is associated with the formation of reactive oxygen intermediates(Reference Murali and Goyal31), suggesting that the scavenging effects of antioxidants on free radicals and the inhibition of lipid peroxidation by antioxidant treatment may play a role in improving renal dysfunction in diabetes.
We examined selective protein expressions in order to investigate the molecular mechanism through which dietary antioxidant supplementation alters the inflammatory response in diabetic nephropathy. Hyperglycaemia causes glycation of various proteins leading to the formation of superoxide radicals (O2−) and thus, increased levels of free radicals are found in diabetes(Reference Signorello, Viviani and Armani32). A previous study has shown that ROS-scavenging enzymes, such as SOD activity, were significantly higher in diabetic rats than in non-diabetic ones(Reference Kim, Cho and Lee33). The result, which is similar to our present study, comes from the phenomenon of physiological adaptation to an increase in ROS scavengers caused by diabetes by means of activated SOD.
HO-1 is induced under a wide variety of conditions associated with oxidative stress and is regarded as a protective response to oxidants(Reference Kruger, Peterson and Schwartzman34). The lower protein expression of HO-1 that we observed in diabetic nephropathy seems to be in contrast to findings of other studies(Reference Kruger, Peterson and Schwartzman34–Reference Hayashi, Haneda and Koya36), which showed an increase in HO-1 protein levels in diabetes and a paradoxical decrease in HO-1 activity. In the present study, we reported that HO-1 protein levels were restored in the dietary antioxidant-supplemented groups. An increase in HO-1 will increase anti-inflammatory and antioxidant capacities.
NF-κB is composed of a family of inducible transcription factors that serve as essential regulators of the host immune and inflammatory response(Reference Yamamoto and Gaynor37). NF-κB activation in the renal tissue of diabetic rats(Reference Iwamoto, Mizuiri and Tanimoto38), as well as the fact that advanced glycation end products induce oxidative stress and activate NF-κB in mesangial cells(Reference Lal, Brismar and Eklof39), has been reported. Other studies have reported that NF-κB binding activity increases in peripheral blood mononuclear cells in uncontrolled diabetic patients(Reference Hofmann, Schiekofer and Kanitz40), and that this activation is oxidative stress sensitive and correlates with the degree of diabetic nephropathy, suggesting that oxidative stress plays an important role in the development of diabetic complications(Reference Mohamed, Bierhaus and Schiekofer41). Furthermore, NF-κB promotes the expression of enzymes which contribute to the pathogenesis of the inflammatory response, including iNOS and COX-2(Reference Pahl42).
CRP is known to be produced in response to inflammatory cytokines, and increased levels are considered as significant inflammatory states(Reference Eklund43). It is known that increased CRP levels are associated with diabetes and its complications such as nephropathy(Reference Goldberg44). The present study showed that dietary antioxidant supplementation regulated the expressions of iNOS, COX-2 and CRP proteins by controlling the expression of pIκBα protein. Particularly, these results showed that interacting with different antioxidants, including NAC, vitamin C and vitamin E, amplified their effects.
Based on the results, ROS generation caused by hyperglycaemia increased oxidative stress and stimulated NF-κB activation in diabetic kidneys and decreased their function by increasing the inflammatory response. We found that a long-term antioxidant cocktail supplementation reduced the degree of diabetes and prevented the progress of diabetic nephropathy. This result encouraged us to believe that a long-term antioxidant cocktail supplementation may have a potential benefit in preventing and improving diabetic nephropathy by regulating renal inflammation. However, further study is required to clarify the mechanisms involved in the development of diabetic complications such as nephropathy. Moreover, further research should be conducted to verify the safe concentration needed for each antioxidant when employed in clinical settings for application to human studies.
Acknowledgements
The present study was supported by a grant from the Kyung Hee University (KHU-20080576). Y. L. conceptualised the research, supervised the laboratory analysis and wrote the manuscript for important intellectual content. N.-Y. P. wrote the manuscript and performed the laboratory work. S.-K. P. performed the laboratory work. All authors reviewed the final manuscript. There are no conflicts of interest to declare.













